Step-by-step guide for endoscopic mitral valve surgery
Introduction
Minimally invasive mitral valve surgery (MIMVS) has gained increasing popularity over the last two decades. In many experienced centers, the minimally invasive approach has become the standard of care for surgical correction of mitral valve (MV) disease, with similar surgical results compared to median sternotomy. Moreover, the limited incision results in reduced postoperative pain, decreased perioperative blood loss and shortening of length of hospital stay (1). Additionally, for reoperations, MIMVS has proven to result in a lower mortality rate with an equal risk of stroke compared to resternotomy (2). Furthermore, from a patient’s point of view, the minimally invasive approach offers the possibility of a superior cosmetic result and faster rehabilitation (3). Different techniques have been described for MIMVS ranging from direct vision, endoscopic- assisted, robotic-assisted to full endoscopic approach. In our tertiary referral center, full endoscopic MIMVS is the preferred surgical approach for MV operations with or without tricuspid valve surgery and arrhythmia surgery. The aim of this manuscript is to provide our method of patient selection and technical details regarding the full endoscopic approach in MV surgery. We also describe our techniques, tips and tricks used for full endoscopic MV repair.
Patient selection and preoperative workup
In general, indications and contraindication for endoscopic MV surgery are relative and dependent on the expertise of each surgeon and center. In our center, relative contraindications for elective non-redo MIMVS include: significant mitral annular calcification, large chest with a distance between the MV annulus and right-sided chest wall of more than 25 cm, extensive pulmonary adhesions, more than grade 1+ aortic valve regurgitation, extensive aortic dilatation, extensive abdominal aortic atherosclerosis or small peripheral arterial diameters relative to patients’ body mass index (BMI) or an absolute peripheral arterial diameter less than 7 millimetre.
All patients with atrioventricular valve disease and non-significant coronary artery disease referred to our center are primarily discussed in our multidisciplinary MV heart-team, consisting of a dedicated MV surgeon, an interventional cardiologist with expertise in transcatheter mitral repair techniques and an imaging cardiologist. The mechanism of mitral regurgitation is clarified through conventional transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE). Additionally, an electrocardiography-triggered computed tomography (CT) angiography of the aortic root and ascending aorta, followed by a high-pitch spiral CT angiography from the aortic arch to the femoral bifurcation is performed to assess eligibility for peripheral cannulation and determine ideal intercostal space (ICS) for a right-sided thoracotomy, as described previously (4). If not done already, patient’s coronary anatomy is fully investigated either with coronary angiography or a CT angiogram and non-surgical lesions are stented if required.
Preparation and anaesthetic set-up
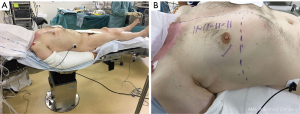
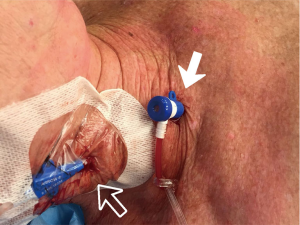
After induction of general anesthesia, selective unilateral lung ventilation is achieved with a double lumen endotracheal tube. In our surgical set-up, the patient is placed in supine position on an operating table that can bend. The patient is positioned with the level of incision at the hinge of the flexible table (Figure 1A,B). The right arm is kept in a slightly flexed position, adjacent to the body and the left arm is tucked in next to the body. The chest is marked with an ultraviolet pen, marking the median sternotomy line, right-sided ribs and intercostal spaces from the second to fifth, level of the diaphragm and the incision. The incision is usually just below the nipple in men and in the infra-mammary fold in women, with degrees of variations depending on each patient’s body habitus. External defibrillator pads are placed posteriorly and anteriorly on the chest wall. Pre-procedurally, the sheath for cannulation of the superior vena cava (SVC) is placed by the anesthesiologist during the insertion of the central line (Figure 2). Periprocedural TEE with 2-dimensional (2D) and 3-dimensional (3D) images are used for evaluation of the prolapsing segments, length measurement of the anterior mitral leaflet, annular dimensions, ventricular function, tricuspid valve assessment as well as assessment of hemodynamics during cardiopulmonary bypass (CPB) and surgical results after weaning from CPB.
Procedure
A right-sided mini-thoracotomy of 4 cm is made in the fourth or fifth intercostal space based on 3D CT reconstruction images. As described earlier, in men, the incision is usually located just below the nipple, while in women the skin incision is made in the infra-mammary fold for cosmetic considerations. An Alexis soft-tissue retractor (Applied Medical, Rancho Santa Margarita, CA, USA) is inserted, preventing extensive rib retraction and reducing postoperative pain. Visualization is accomplished by a 10 mm 30° 3D endoscope (Karl Storz Endoscopy-America, Culver City, CA, USA) that is placed through a trocar in the same ICS as the mini-thoracotomy. Visualization is optimized by use of dedicated 3D glasses and a 3D monitor, which is placed at eyelevel of the surgeon (Figure 3).
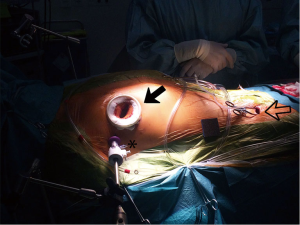
Cannulation and clamping
Arterial cannulation options for MIMVS consist of femoral arterial, axillary arterial or direct ascending aortic cannulation (5,6). In our center, the preferred cannulation strategy consists of peripheral femoral arterial and venous cannulation in the groin under TEE guidance and subsequent use of retrograde perfusion. The CT, made in the preoperative work-up, provides valuable information regarding vessel quality and trajectory and is therefore made in all patients considered eligible for MIMVS. Additionally, dedicated software (Fujifilm Synapse Vincent system, Fujifilm Corporation, Tokyo, Japan), is used to determine the optimal cannula size preoperatively (7). Preferably, in absence of aorto-iliac atherosclerotic disease and in case of adequate vessel size, the right groin is used. We perform a small 2–3 cm incision horizontally over the femoral vessels around 2 cm below the inguinal ligament. We take extra care to avoid entering the lymph node bundle situated over the femoral sheath by retracting it medially. After the exposure and insertion of the purse strings on femoral vessels, SVC is first cannulated using the sheath that was inserted by the anesthesiologist. Then the femoral vein is cannulated using the TEE bicaval view. It is important to ensure that both cannulas are placed in the correct position at the entrance of cava in the right atrium for optimal drainage. Afterwards, the descending aorta is visualized with TEE and the femoral artery is cannulated.
In our practice, aortic occlusion is accomplished either with transthoracic aorta clamping (Chitwoord DeBakey-clamp, Scanlan International, Inc., St Paul, MN, USA) or endo-aortic occlusion by use of an endo-aortic balloon (IntraClude, Edwards Lifesciences, Irvine, CA, USA), as described previously (8). The former is used if the ascending aorta diameter is around 4 cm or more as in these cases endo-aortic balloon occlusion could potentially be unfeasible, resulting in suboptimal myocardial protection (9). The transthoracic aorta clamp is placed through a separate skin incision in the intercostal space just above and lateral to the mini-thoracotomy incision. In case the external clamp is used, a transaortic needle for cardioplegia delivery is introduced. Endo-aortic balloon occlusion is our preferred strategy since 2017. The triple lumen catheter of the endo-balloon has the advantages of combined cardioplegia delivery, venting, root pressure monitoring and occlusion. In patients referred for reoperations with unsuitable aorta dimension’s for endo-aortic balloon occlusion, a third strategy can be used to avoid dissection of the adhesions around the ascending aorta; these patients are cooled to 28 °C and ventricular fibrillation arrest (VF) is induced.
Exposure
Once on cardiopulmonary bypass, the pericardium can be opened safely. We open the pericardium anterior to the phrenic nerve, starting from the level of the ascending aorta to the level of the diaphragm (Figure 4). Multiple pericardial sutures are placed for retraction of the pericardium to achieve optimal exposure. Before aortic occlusion, a stab incision is made parasternally for the placement of the external part of the atrial retractor (HV retractor, USB Medical, Huntingdon Valley, PA, USA).

In case of use of the transthoracic aortic clamp, a sucker is used to lift the transverse sinus to prevent iatrogenic lesions of left atrial appendage while clamping (Figure 4). When conducting this standard maneuver, one should be careful in manipulation of the ascending aorta, as we have observed a case of iatrogenic antegrade type A aortic dissection in our early experience (11). As mentioned above, in complex reoperations under VF patient is cooled to 28 °C and dissection of the Waterstone’s groove is continued (Figure 4). The disadvantages of operating under VF could be a suboptimal exposure of the valve due to backflow.
The left atrium is then opened and the atrial wall is retracted anteriorly by inserting the blade of the atrial retractor and connecting it to the shaft of the outside table-mounted holder. The blade size can be determined preoperatively, which is based on left atrial volume. A sump sucker connected to an additional vent is placed in the left atrium for adequate drainage of the operation field (Figure 4).
Upon visualization of the MV, nerve hook testing is performed for evaluation of the exact valvular pathology. According to the segmental analysis of Carpentier, the P1 region of the posterior leaflet is used for reference. In case the P1 region is involved in the mechanism of regurgitation, any other unaffected segment or the annular level can be used as the reference point (Figure 5). The principle of valvular analysis is based on confirmation of the pathology as seen on preoperative TEE.

MV repair
MV repair consists of a plethora of different techniques. In MV prolapse, after a possible resection, neochordae are placed for correction of the prolapsing segments and the coaptation defect. To achieve an optimal result, a coaptation surface of at least 8 to 10 mm is warranted, confirmed by the ink test. In patients with commissural prolapse, papillary muscle reposition can be performed to eliminate the commissural prolapse. For this technique, a single suture with pledgets is placed through the head of the intermediate papillary muscle. The intermediate head is then freed from the anterior head and fixated to the posterior head, resulting in a reduction of the commissure (Figure 5).
Almost all of our mitral repair patients receive artificial neochordae for correction of mitral regurgitation, as described by Falk et al. (13). These neochordae are placed with respect to the midline of the MV and the papillary muscles, therefore no neochordae should cross the midline. Neochordae are placed through the intermediate heads of the papillary muscles. Neochordae through the posteromedial papillary muscles are placed in backhand position and through the anterolateral papillary muscles in forehand position (Figure 5). The neochordae are fixated on the free edge of the MV leaflet by locking both sutures and knotting the ends in the middle of both insertions. Other techniques for patients with prolapse include, triangular and quadrangular resection in patients with Barlow disease and excessive leaflet tissue, the double-orifice technique as described by Alfieri et al. (14) and/or closure of clefts and indentations.
After placement of the neochordae, an annuloplasty ring is implanted to support the repair. Annular ring size is determined by the length of the anterior MV leaflet. As the incision in our center is limited, we do not use conventional ring sizers, but size the valve with a flexible sizer cutout of sterile paper. The annuloplasty sutures are placed according to a suture map and sequence, which is published elsewhere (15). Briefly, the sutures start clockwise at the posteromedial commissure were one suture is placed in reversed forehand position. Secondly four sutures are placed counterclockwise at the anterior annulus in backhand position. From the anterolateral commissure onwards, three sutures are placed in reversed backhand position. To close the ring four sutures are placed clockwise in forehand position (Figure 5). The suture map allows surgeons to place the sutures without blocking the endoscopic vision. The sutures are tightened with either a knot pusher or an automated fastener (COR-KNOT, LSI SOLUTIONS, Victor, NY USA).
Closure
Once sufficient coaptation is achieved and we are satisfied with the saline test the de-airing process is initiated. In case with endo-aortic balloon occlusion, a vent is placed through the MV and the patient is positioned in a Trendelenburg position, to start the de-airing process before the atrium is closed. In case with the use of Chitwood clamp, patient is positioned in anti-Trendelenburg position and de-air is achieved through the root vent. Throughout the whole operation, CO2 was insufflated. The atrium is closed with double running 4-0 polypropylene sutures. Before declamping, a pacemaker wire is placed on the anterior wall of the left ventricle. After decannulation, heparin is antagonized for promotion of hemostasis. Before closing, the right lung is once more deflated for a final evaluation of the left atrial suture line for bleeding, after which a single 10mm drain is placed through the camera port.
Tips, tricks and pitfalls
- In patients with an elevated hemi diaphragm a suture at the caudal part of the pericardium can be placed through a tourniquet and retracted out of the patient, thereby lowering the diaphragm without blocking of the endoscopic view for the surgeon (Figure 6).
 Figure 6 Tips, tricks and pitfalls (16). Available online: http://www.asvide.com/article/view/30542
Figure 6 Tips, tricks and pitfalls (16). Available online: http://www.asvide.com/article/view/30542 - In MIMVS, optimal exposure is crucial for a successful procedure. We use a paper ruler for optimal subvalvular exposure. The basis of the ring is a paper ruler (Purple Surgical, Shenley, England), which is folded into a ring and fixed with small vascular clips. The ring is inserted into the MV annulus to retract the leaflets and create maximum visual exposure. The paper ring allows surgeons to visualize the subvalvular apparatus and the ventricular cavity to implant artificial chords more easily (Figures 6,7
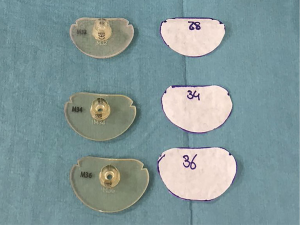
). - Sizing the annulus with a normal sizer can be difficult in MIMVS due to the limited size of the incision. Therefore, the outline of the sizer is transferred on to paper and trimmed by the scrub nurse prior to incision. These paper sizers can be easily put in to place and resemble the exact same outline as the original sizer (Figures 6,8
). - Saline testing can be used in MV surgery to evaluate the repair intra-operatively. In MV repair for isolated posterior leaflet prolapse, saline testing sometimes shows an unexpected full prolapse of the anterior leaflet, which was not present on preoperative echocardiography. Several intra-operatively maneuvers can counter this phenomenon, such as: pulling down the diaphragm, repositioning of the atrial retractor or forcing down the anterior part of the annuloplasty ring. Despite these maneuvers, some patients still maintain a prolapse of the anterior leaflet during saline testing. When saline testing shows an unexpected prolapse of the anterior leaflet, not present on preoperative echocardiography, no additional surgical techniques should be performed to achieve an excellent postoperative result (17) (Figure 6).
Role of team members
The role of dedicated heart teams, including scrub nurses, anesthesiologist, perfusionist and cardiologist should not be underestimated (18). In our center, the day prior to the operation, patients are discussed with the whole team and the team is informed about the surgical approach, cannula sizes and potential technical difficulties. During the operation, the communication between the surgeon, the anesthesiologist and perfusionist is crucial, especially in case of use of the endo-aortic balloon. Additionally, in most cases, the preoperative TEE is performed by the same imaging cardiologist as the intraoperative echocardiogram directly after weaning from bypass, limiting the inter-observer variability and ensuring an unbiased evaluation of the surgical result.
Conclusions
Starting a MIMVS program requires a step-by-step approach; correct patient selection and patient preparation especially during the early phases, patience to deal with the learning curve, low threshold in converting to median sternotomy specifically during the initial cases to avoid catastrophe, diligence myocardial protection strategies, team work and strict adherence to the procedural and surgical steps. In addition, surgical techniques used for conventional open surgery cannot be directly adopted in MIMVS due to the increased distance from the chest wall to the MV, the limited operative space, decreased surgical maneuverability, the use of long-shafted instruments, and the need for video assistance. Adaptation of existing techniques combined with extensive preoperative planning and working with dedicated teams could help starting surgeons to select patients for MIMVS, minimalize postoperative complications and improve patient outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Minimally Invasive Mitral Valve Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.02.02). The series “Minimally Invasive Mitral Valve Surgery” was commissioned by the editorial office without any funding or sponsorship. PSV served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent for the usage of the video material was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52. [Crossref] [PubMed]
- Daemen JH, Heuts S, Olsthoorn JR, et al. Right minithoracotomy versus median sternotomy for reoperative mitral valve surgery: a systematic review and meta-analysis of observational studies. Eur J Cardiothorac Surg 2018;54:817-25. [Crossref] [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [Crossref] [PubMed]
- Heuts S, Maessen JG, Sardari Nia P. Preoperative planning of left-sided valve surgery with 3D computed tomography reconstruction models: sternotomy or a minimally invasive approach? Interact Cardiovasc Thorac Surg 2016;22:587-93. [Crossref] [PubMed]
- Glauber M, Murzi M, Solinas M. Central aortic cannulation for minimally invasive mitral valve surgery through right minithoracotomy. Ann Cardiothorac Surg 2013;2:839-40. [PubMed]
- Grossi EA, Loulmet DF, Schwartz CF, et al. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg 2012;143:S68-70. [Crossref] [PubMed]
- Heuts S, Olsthoorn J, Maessen J, et al. Planning minimally invasive mitral valve surgery. J Vis Surg 2018;4:212. [Crossref]
- Bentala M, Heuts S, Vos R, et al. Comparing the endo-aortic balloon and the external aortic clamp in minimally invasive mitral valve surgery. Interact Cardiovasc Thorac Surg 2015;21:359-65. [Crossref] [PubMed]
- Czesla M, Mogilansky C, Balan R, et al. Evolution of a minimally invasive mitral valve program. J Vis Surg 2016;2:169. [Crossref] [PubMed]
- Olsthoorn JR, Heuts S, Attaran S, et al. Visual exposure. Asvide 2019;6:073. Available online: http://www.asvide.com/article/view/30540
- Hermans S, Heuts S, Olsthoorn J, et al. Antegrade type A aortic dissection under endoscopic vision during minimally invasive mitral valve repair: a case report. J Vis Surg 2018;4:211. [Crossref]
- Olsthoorn JR, Heuts S, Attaran S, et al. Mitral valve repair. Asvide 2019;6:074. Available online: http://www.asvide.com/article/view/30541
- Falk V, Seeburger J, Czesla M, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg 2008;136:1205-discussion 1205-6. [Crossref] [PubMed]
- Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001;122:674-81. [Crossref] [PubMed]
- Sardari Nia P, Olsthoorn J, Heuts S, et al. Suturing map for endoscopic mitral valve repair developed on high-fidelity endoscopic simulator. Multimed Man Cardiothorac Surg 2018;2018.
- Olsthoorn JR, Heuts S, Attaran S, et al. Tips, tricks and pitfalls. Asvide 2019;6:075. Available online: http://www.asvide.com/article/view/30542
- Olsthoorn JR, Heuts S, Streukens SAF, et al. Unexpected prolapse of the anterior leaflet during saline testing in mitral valve repair. Eur J Cardiothorac Surg 2018; [Epub ahead of print]. [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
Cite this article as: Olsthoorn JR, Heuts S, Attaran S, Cornelissen S, Maessen JG, Nia PS. Step-by-step guide for endoscopic mitral valve surgery. J Vis Surg 2019;5:30.