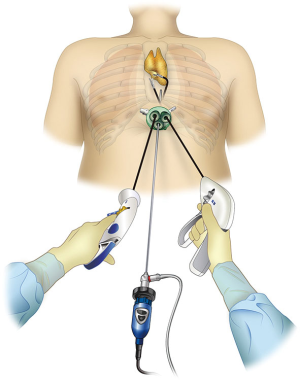
Thymectomy with an endoscopic approach
Introduction
Various surgical approaches, including endoscopic surgery, are used when operating on tumors of the anterior mediastinum. Surgical methods involving an anterior mediastinum approach anatomically involve either making a vertical incision in the sternum or approaching from the cervical, lateral chest, or abdominal region. Accordingly, surgical approaches for anterior mediastinal tumors include conventional median sternotomy, transcervical thymectomy from the cervical region, lateral thoracic intercostal approach, and the subxiphoid approach (1-4). Recently, robot-assisted surgery and single-port surgery are now performed as new forms of endoscopic surgery in the field of thoracic surgery and are now being adapted for anterior mediastinal tumors (4-7). In this report, we will review current endoscopic surgical approaches for anterior mediastinal tumors and describe surgical techniques for thymectomy by a lateral thoracic intercostal approach, which is currently widely performed, as well as thymectomy by a subxiphoid approach.
Median sternotomy
Median sternotomy is a classic method that was first reported by Milton et al. in 1897. This technique is still the standard surgical approach for anterior mediastinal tumors (8). An incision of approximately 20 cm is made from the cervical region of the precordium, and the sternum is cut with a sternal saw. A rib retractor is then used to expose the chest for surgery.
As for the advantages of this approach, a superior surgical field can be secured for the surgeon, and surgical operations including suturing can be performed without any limitations. This approach can be used for the mediastinum and lungs. Disadvantages include poor cosmetic outcome and cutting the sternum is associated with the risk of sternal osteomyelitis owing to wound infection. Infection of the sternum occurs in 1–2% of cases (9). Many reports have also indicated that pain is more intense than that associated with the endoscopic lateral thoracic intercostal approach and that more time is needed for the patient to return to their normal lifestyle postoperatively (10,11). However, this surgical approach has the widest range of surgical indications, and it can be performed even for cases in which the tumor has invaded adjacent organs.
Transcervical thymectomy
This method involves an approach from the cervical region. It was reported in 1912 by Schumacher and Roth as a technique for thymectomy for myasthenia gravis (12). In 1988, Cooper et al. developed a specialized retractor and proposed a method of improving the surgical field (1). They reported that surgical outcomes were comparable with those in median sternotomy (13). The advantages of this surgical technique include the fact that there is no cutting of the sternum, no intercostal approach is required, intercostal neuralgia is therefore not felt, and pain is minimized. The disadvantages include the fact that patients do not like that an incision is made in the neck region, which cannot be hidden by clothing; although total thymectomy is possible, it is difficult to secure the surgical field, and surgical operations are limited even if the surgeon is familiar with the procedure. This technique is challenging when used to remove large tumors or for combined resection of adjacent organs.
Lateral thoracic intercostal approach
The lateral thoracic intercostal approach was reported by Landreneau et al. in 1992. It is currently the most widely used surgical approach for endoscopic thymectomy worldwide (2). For its advantages, cutting of the sternum can be avoided and that the wound is in the lateral chest region and is therefore not prominent, allowing for excellent cosmetic outcomes. However, disadvantages include occurrence of intercostal neuralgia, with pain lasting for 1–2 months. Moreover, in approximately 10% of these cases, the patient suffers from pain and numbness associated with a condition called post-thoracotomy pain syndrome throughout their lives. Disadvantages associated with the surgical technique include the difficulty in securing a visual field in the upper pole of the thymus and difficulty in confirming the site of the opposing phrenic nerve from one side of the lateral chest.
Lateral thoracic approach for thymectomy
The approach may be taken from the left or the right side. Approaching from the right side offers a better view of the cervical region compared with that from the left side. Although approaching from the left makes it possible to resect the fatty tissue on the pericardium while confirming the left phrenic nerve, the visual field in the cervical region is poor. However, when the patient is in the supine position and the left approach is taken, the fatty tissue on the pericardium is difficult to observe because it is too close to the port.
This can be significantly improved by CO2 insufflation. Although the author now barely uses this approach owing to the significant advantages of the subxiphoid approach, when this approach is taken, it is taken from the right because it is associated with a relatively good visual field of the cervical region and enables observation of the left brachiocephalic vein. This approach is indicated for patients eligible for thymectomy and patients showing no invasion of the brachiocephalic vein. If invasion of the left brachiocephalic vein is suspected, the patient is considered not eligible for this approach owing to the difficulty in securing the blood flow. In cases of pericardial invasion, patch closure of the pericardium may be possible depending on the site.
Surgical technique
Differential lung ventilation is performed with the patient under general anesthesia. The patient is placed in the supine position. When thymectomy is performed with the lateral thoracic approach, the patient should be placed in the supine position or 30° semi-supine position. If bleeding occurs from the left brachiocephalic vein, a site of the left brachiocephalic vein peripheral from the bleeding site can be clamped. It is best to place the patient in the supine position or 30° semi-supine position because these positions make it possible to quickly perform median sternotomy in case of bleeding. A surgical bed is used to elevate the trunk higher than the operating table, while lowering the right arm and placing the same alongside the trunk. CO2 insufflation may be performed. When approaching from the right, the surgeon stands on the right side of the patient; the assistant is also on the right side of the patient further to the cranial side than the surgeon, and the scopist stands caudally from the surgeon. The nurse stands on the left side of the patient.
Ports are inserted from the third intercostal anterior axillary line, fifth intercostal anterior axillary line, and sixth intercostal anterior axillary line. The assistant’s port is inserted between the fifth and sixth intercostal anterior axillary lines (Figure 1). CO2 insufflation is then performed. A vessel-sealing device is used to resect the mediastinal pleura 1 cm anteriorly from the right phrenic nerve from the diaphragm to the right internal thoracic vein. The mediastinal pleura on the back surface of the sternum is also resected and joined to the mediastinal pleura incision site. The thymus is separated from the back surface of the sternum. The surgeon can then observe the protrusion of the lung on the contralateral side. Care should be taken so that it is not damaged by opening the thoracic cavity on the contralateral side. The lower pole of the thymus is separated from the left pleura, back surface of the sternum, and pericardium. The right internal thoracic vein is separated from the surrounding fatty tissue and transected with the vessel-sealing device. This improves the visual field of the cervical region. The left brachiocephalic vein is separated from the fatty tissue, and its position is confirmed. The upper right pole of the thymus is grasped and separated from the brachiocephalic artery, after which the left upper pole of the thymus is grasped and separated from the trachea. This is done carefully to avoid damaging the inferior thyroid vein that runs between the upper pole and trachea. The inferior thyroid vein may be also transected. The cervical thymus is pulled caudally, and the left brachiocephalic vein is separated from the thymic tissue. The thymic vein is transected with the vessel-sealing device when appropriate, and the thymus is extracted (Figure 2). When taking the lateral thoracic approach with Da Vinci robot-assisted surgery, the patient is placed in the supine position with a 30° slant. Ports are inserted anteriorly from the third, fifth (camera), and sixth intercostal anterior axillary lines. The assistant’s port is inserted between the fifth and sixth intercostal anterior axillary lines. Generally, it is best to perform CO2 insufflation because this can significantly improve the visual field. The third intercostal port should be placed quite laterally because it will otherwise likely become too close to the mediastinum.
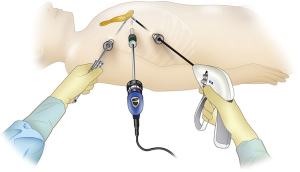

Subxiphoid approach
Recently, the subxiphoid approach has been reconsidered. This approach, which was reported by Kido et al. in 1999, involves resecting thymic and anterior mediastinal tumors from a subxiphoid incision (15). In 2012, we reported on single-port thymectomy using CO2 insufflation (4) (Figure 3). This approach, which involves performing surgery from a single subxiphoid incision, does not cause intercostal neuralgia because intercostal approach is not taken. Moreover, it causes only slight pain and offers a superior cosmetic result (16). In addition to single-port surgery, a dual-port approach, in which device interference is avoided by adding another port to the lateral intercostal region, and a robot-assisted surgery approach, involving additional ports at bilateral intercostal sites, have also been reported (7,17) (Figure 4). The author performs the single-port thymectomy with the subxiphoid approach for cases of thymoma with no invasion or myasthenia gravis with no tumor owing to the low level of invasion. Even if the tumor has invaded a lung, this approach can be used for up to partial lung resection. The disadvantages of single-port thymectomy with the subxiphoid approach include the difficulty in performing the technique. Suturing from the single subxiphoid incision is challenging. In cases in which pericardial invasion is suspected and suturing is required to fill the pericardial defect region, dual-port thymectomy or robot-assisted surgery, in which surgical operability has been improved by adding an intercostal port in single-port surgery, is indicated. For cases in which the thymoma has invaded the surrounding organs, the technique is selected depending on the surrounding invasion.
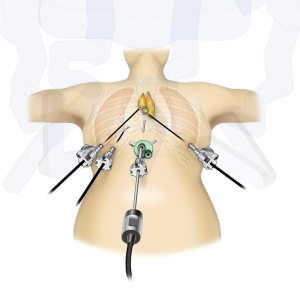
Surgical technique
In the single-port thymectomy technique, the surgeon stands to the right side of the patient and places a 3-cm horizontal incision along the Langer line 1 cm caudally from below the xiphoid process. Although it is possible to make a vertical incision, a horizontal incision reduces camera and forceps interference and makes it easier to secure the scopist’s surgical field. A vertical incision is advantageous in large tumors because the vertical incision can make it easier to expand the incision downward for the removal of the tumor.
The Linea alba and abdominal rectus muscle enthesis are transected to reach the back surface of the xiphoid process. The thymus is blindly separated from the back surface of the sternum by the surgeon. An approximately 1-cm vertical incision is made on the caudal side of the white line. A space for inserting the port during single-port surgery is made blindly by the surgeon, while taking care not to open the peritoneum. Resection of the xiphoid process is not required. The GelPOINT Mini (Applied Medical, Rancho Santa Margarita, CA, USA), a port used for single-port surgery, is inserted into the incision below the xiphoid process. A platform with three inserted sub-ports is fitted to the site, and the surgeon moves to stand between the patient’s legs. The scopist stands on the right side of the patient. CO2 insufflation is then performed at 8 mmHg. A LigaSureTM Maryland sealer (Covidien, Mansfield, MA, USA) is used to transect the thymus from the back surface of the sternum when LigaSureTM, the tip of which does not bend, cannot reach the back surface of the sternum. Ablation is performed using an SILS dissector or SILS scissors (Covidien, Mansfield, MA, USA), both of which have bendable tips. Positive pressure is induced by CO2 insufflation, resulting in ablation and expansion of the space on the back surface of the sternum. The thymus is separated from the sternum up to the cervical area. Then, both sides of the mediastinal pleura are opened, and both sides of the thoracic cavity are exposed. The mediastinal pleura 1 cm anterior from the left phrenic nerve is resected on the cranial side, while separating the lower left pole of the thymus from the pericardium. The back surface of the sternum is connected to the resection line of the mediastinal pleura. Once the surgeon approaches the left brachiocephalic vein, the veins are exposed while lightly weakening them from the cranial side and patient’s anterior side. Once the left brachiocephalic vein site is confirmed, the cranial side of the peripheral left brachiocephalic vein is exposed. This becomes the left border for the resected cervical thymus. Then, the mediastinal pleura 1 cm anterior from the right phrenic nerve is resected on the cranial side while separating the right lower pole of the thymus from the pericardium. The back surface of the sternum is then connected to the mediastinal pleura resection line. By separating the right internal thoracic vein from the surrounding fatty tissue to expose it, the left brachiocephalic vein is exposed. Surgeries in the cervical region then commence. The inner border of the right brachiocephalic vein should be confirmed. The right internal thoracic vein is not normally transected. The upper pole of the thymus is grasped with grasper forceps and pulled caudally to push down on the left brachiocephalic vein, thereby improving the visual field in the cervical region. The upper thymus pole and fatty tissue in the cervical region are grasped with a clinch. Then, while shaking the clinch to the left and right as necessary, it is separated from the right brachiocephalic vein on the right side, the thyroid gland on the upper edge, the brachiocephalic artery and trachea on the dorsal side, and the upper edge of the left brachiocephalic vein on the left side (already separated). Once the cervical thymus has been freed, it is pulled on one lateral side to expose the entire left brachiocephalic vein. While doing this, the thymic vein is successively transected with LigaSureTM to completely resect the thymus. The thymus is then extracted via the subxiphoid incision. A 20-Fr drain is inserted into the subxiphoid incision, and the wound is closed (Figure 5).

Comments
When endoscopic surgery is adapted for thymectomy, three possible approaches can be taken, namely, the cervical approach, the lateral thoracic approach, or the subxiphoid approach. The endoscopic surgery approach is selected based on the patient status, tumor location and size, surgical operability and invasion, and the surgeon’s viewpoint and level of experience. Single-port and robot-assisted surgeries are now being performed. Although single-port surgery is associated with limited surgical operability, it offers excellent cosmetic outcomes. Moreover, because pain can be minimized with both the intercostal and subxiphoid approaches, less invasive surgery can be performed. Owing to the fact that robot-assisted surgery involves the insertion of a robotic arm, a greater number of ports are required. However, the robot forceps joints can move similar to the human wrist through the robot system, physiological tremors can be eliminated, and the 3D vision surgical field greatly improves surgical operability.
When endoscopic surgery is adapted for thymectomy, the surgeon must understand the advantages and disadvantages of each approach and consider eligibility for single-port or robot-assisted surgery to determine which approach will offer the greatest benefit to the patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Jose Luis Danguilan) for the series “Dedicated to the 6th Asian Single-port VATS Symposium 2018” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.03.09). The series “Dedicated to the 6th Asian Single-port VATS Symposium 2018” was commissioned by the editorial office without any funding or sponsorship. TS serves as an unpaid editorial board member of Journal of Visualized Surgery from Feb 2018 to Jan 2020. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Landreneau RJ, Dowling RD, Castillo WM, et al. Thoracoscopic resection of an anterior mediastinal tumor. Ann Thorac Surg 1992;54:142-4. [Crossref] [PubMed]
- Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Ashton RC Jr, McGinnis KM, Connery CP, et al. Totally endoscopic robotic thymectomy for myasthenia gravis. Ann Thorac Surg 2003;75:569-71. [Crossref] [PubMed]
- Rückert JC, Ismail M, Swierzy M, et al. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann N Y Acad Sci 2008;1132:329-35. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Milton H. Mediastinal surgery. Lancet 1897;1:872-5. [Crossref]
- Demmy TL, Park SB, Liebler GA, et al. Recent experience with major sternal wound complications. Ann Thorac Surg 1990;49:458-62. [Crossref] [PubMed]
- Grossi E1. Comparison of post-operative pain, stress response, and quality of life in port access vs. standard sternotomy coronary bypass patients. Eur J Cardiothorac Surg 1999;16:S39-42. [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Schumacher ED, Roth O. Thymekyomie bei einem Fall von Morbus Basedowi mit Myastenie. Mitteil Grenzgebiet Med Chirurg 1912;25:746.
- Shrager JB, Deeb ME, Mick R, et al. Transcervical thymectomy for myasthenia gravis achieves results comparable to thymectomy by sternotomy. Ann Thorac Surg 2002;74:320-6; discussion 326-7. [Crossref] [PubMed]
- Suda T. Thymectomy with the lateral thoracic approach. Asvide 2019;6:071. Available online: http://www.asvide.com/article/view/30522
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49:i54-8. [PubMed]
- Suda T, Ashikari S, Tochii D, et al. Dual-port thymectomy using subxiphoid approach. Gen Thorac Cardiovasc Surg 2014;62:570-2. [Crossref] [PubMed]
- Suda T. Thymectomy with the subxiphoid approach. Asvide 2019;6:072. Available online: http://www.asvide.com/article/view/30523
Cite this article as: Suda T. Thymectomy with an endoscopic approach. J Vis Surg 2019;5:29.