Special issue on inflammatory thoracic disease: surgical experience of pulmonary aspergilloma
Introduction
Aspergillus organisms tend to colonize preexisting lung cavities, including healed tuberculous lesions, benign lung abscess, and bronchiectasis, that may lead to the development of aspergilloma requiring surgical treatment (1). However, given that surgical resection is associated with high complication and mortality rate, it is usually performed reluctantly. Shortly after its introduction, video-assisted thoracic surgery (VATS) was used to attempted the removal of pulmonary aspergilloma (PA). Traditionally, severe and tight adhesion, calcified lymph node around major vessels, massive intraoperative bleeding, and spillage to the pleural cavity or other lobes have been the main and frequent complications that arise during the operation. VATS has proven to have several advantages over open thoracotomy, including less postoperative pain, better recovery of pulmonary function, and superior cosmetic outcome (2). All things considered, VATS procedure for aspergilloma has been gaining significant popularity. For instance, as a preventive measure, pulmonologists refer patients to thoracic surgeons, even for cases that are not severe. The purpose of this study was to provide a review of the surgical strategies and methods used to resect PA in our institution.
Methods
Patients
Between 2003 and 2018, 76 patients were surgically treated for PA at Seoul National University Bundang Hospital (SNUBH). Surgical indications were based on symptoms, including recurrent hemoptysis failed by bronchial artery embolization (BAE), persistent cough, and intractable sputum.
Diagnosis
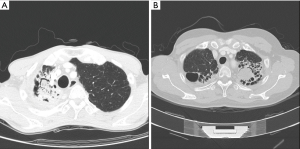
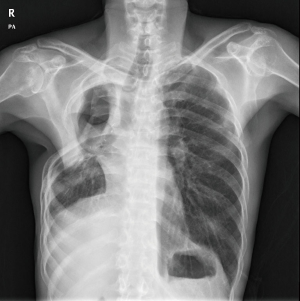
PA was frequently found with an underlying pulmonary disease, such as pulmonary tuberculosis and bronchiectasis (Figure 1A,B). Figure 1A shows a patient who was diagnosed with pulmonary tuberculosis 5 years ago, and who, after anti-tuberculosis medication, experienced negative conversion of sputum acid-fast bacillus (AFB) with a destructed right upper lobe (RUL). Six months before surgery, the patient suffered from recurrent hemoptysis and underwent BAE two times. Computed tomography (CT) showed some calcification and meniscus sign on the RUL. Figure 1B shows underlying bronchiectasis on the bilateral upper lobes and aspergilloma on the left upper lobe (LUL). However, the patient did not complain of hemoptysis, but suffered from recurrent pneumonia with intractable sputum and cough.
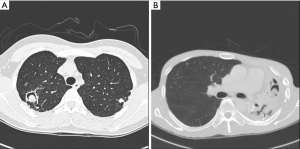
PA was divided into simple or complex types based on chest CT image (Figure 2A,B). Figure 2A shows the simple PA type on the RUL, showing no underlying parenchymal destruction. Simple PA can usually be resected by minimally invasive surgery. Complex PA, on the other hand, is frequently accompanied by another chronic inflammatory lung disease, such as pulmonary tuberculosis or bronchiectasis. Figure 2B shows an LUL that is almost entirely destructed, with decreased volume.
Preoperative evaluation and management
To predict the postoperative pulmonary function, a preoperative pulmonary function test (PFT) was performed; however, patients with a history of persistent hemoptysis or pulmonary tuberculosis were contraindicated to receive PFT. For these patients, an arterial blood gas analysis was performed instead. For patients with poor lung function, a preoperative lung perfusion scan was used to decide the feasibility of resection or extent of resection. However, because the lobe that would be resected was already destroyed, postoperative patients’ pulmonary function did not deteriorate after resection of the destroyed lobe. Depending on the condition of patients, we provided preoperative pulmonary rehabilitation and nutritional support.
Operative technique
For anesthesia, isolation of the affected lung was very important to prevent spillage and contamination to the contralateral lung and other ipsilateral lobes. Hence, if the lesion was located on the RUL, left side double lumen tube (DLT) was inserted to isolate the right lung and a blocker was sometimes inserted to block the RUL to prevent contamination of the right middle and lower lobes. Tracheal suction was frequently needed throughout the operation. After decubitus position was achieved, DLT and blocker position was re-confirmed using bronchoscopy. The use of VATS was dependent on the type of PA. All cases were initiated with a thoracoscopic examination; then, if adhesion was not tight and focal, VATS procedure was considered. However, if adhesion was tight or there was bleeding during dissection causing blurred vision, VATS was converted to open thoracotomy, even though VATS may be needed to aid adhesiolysis. After total adhesiolysis, bleeding control was critical in maintaining stable vital signs and reducing transfusion. Among the various methods of bleeding control, coagulation of bleeding focus and the use of coagulant materials, such as Hemoblock, SURGICEL® (Ethicon), TachoComb (Nycomed, Zurich), in addition to compression using gauze, were effective. Procedural components, including the sequence, dissection, and division of pulmonary vein (PV), pulmonary artery, and bronchus were nearly identical as those of lobectomy for lung cancer (Figure 3). This video shows VATS RUL lobectomy for simple PA. During surgery, handling of the affected lung was minimized as much as possible, and extrapleural dissection was sometimes needed to prevent a rupture of the infected cavity. If the fissure was incomplete, and a small vein was engorged around the fissure, the division of the PV was performed as the last step, because division of the PV may impact lung congestion and result in a poor vision of field. With respect to the dissection and division of the bronchus, clearing the peribronchial tissues by using an energy device, such as LigasureTM (Medtronic), Harmonic Scalpel (Ethicon), and Thunderbeat (Olympus) was considered safe, resulting in minimal bleeding. After resection, regardless of whether there is a rupture of the cavity, irrigation of thoracic cavity, using betadine solution, may be inevitable. Two chest tubes were generally inserted, and an additional chest tube or small drain may be necessary to prevent wound contamination.

Postoperative management
Mechanical ventilation was sustained to stabilize vital signs. Before extubation, portable bronchoscopic evaluation was usually performed for toileting and evaluating the bronchial stump. After extubation, ward ambulation and pulmonary rehabilitation was mandatory for rapid recovery and improvement of pulmonary function. The most common complication, persistent air leak, was difficult to manage. The injured lung was meticulously sutured intraoperatively, and artificial materials, such as Neoveil sheet (Gunze) and BioGlue, were applied. The correct positioning of chest tubes was important to induce effective drainage of pleural fluid and air leak. After surgery, chest tubes were usually applied to thoracic suction, and chemical pleurodesis was performed using fibrin, autologous blood, or chemoagents for persistent airleak.
Results
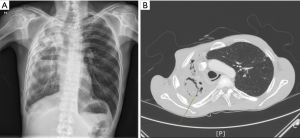
A total of 76 patients, consisting of 35 males and 41 females, were included. The median age was 56 (range, 17–79) years. Hemoptysis was seen in 58 (76%) patients, persistent cough with blood-tinged sputum in 8 (11%) patients, and persistent sputum with cough in 10 (13%) patients. Preoperative BAE was performed in 18 (24%) patients, and other patients with hemoptysis were medicated with oral coagulant agents. With respect to other underlying diseases, pulmonary tuberculosis was present in 29 (38%) patients, bronchiectasis in 16 (21%) patients, and no definite underlying disease in 31 (41%) patients. On chest CT, simple PA was seen in 59 (78%) patients and complex PA in 17 (22%) patients. Lesions were located on the RUL in 31 (41%) patients, the right middle lobe (RML) in 1 (1%) patient, the right lower lobe (RLL) in 13 (17%) patients, the LUL in 17 (22%) patients, and the left lower lobe (LLL) in 14 (18%) patients; most of the lesions (63%) were located in the upper lobes. Regarding surgery, open thoracotomy was performed in 38 (50%) patients and VATS in 38 (50%) patients. All complex PA patients were performed on by open thoracotomy or conversion. Table 1 shows a comparison between simple and complex aspergilloma. Except in 10 patients, all patients had some intraoperative adhesion to the thoracic cavity, with whole-lung adhesion occurring in 38 patients and partial-around-lesion adhesion occurring in 28 patients. Regarding the severity of adhesion, 40 (53%) patients had tight adhesion around the mass, resulting in some patients needing to undergo dissection of the extrapleural space. Bronchial stump coverage was performed only in 20 (26%) patients with viable tissue, such as intercostal muscle or pericardial fat. Intraoperative bleeding developed in 6 (8%) patients who needed to convert to open thoracotomy. Intraoperative spillage of the cavity developed in 4 (5%) patients. As for the extent of resection, lobectomy was done in 44 patients, bilobectomy in 1 patient, and pneumonectomy in 4 patients. Sublobar resection, consisting of 13 wedge resections and 14 of segmentectomies, was also performed in 27 patients. The mean size of the lesion was 3.2 (range, 1.0–90) cm. Major complications developed in 13 (17%) patients. Postoperative empyema was observed in 4 patients. One patient had complex PA underlying pulmonary tuberculosis (Figure 4A,B). Initially, this patient’s right hemithorax was collapsed due to long-term inflammation. The lesion was located on the RUL and inflammation was spread to the RML; thus, right upper bilobectomy was performed despite the presence of some inflammation on the RLL. After bilobectomy, massive air leak continued for 10 days, despite several attempts with chemical pleurodesis to stop it. After that, the clarity of drain changed to turbid and dead space persisted. Exploration showed no major bronchopulmonary fistula, but revealed several parenchymal defects (Figure 5). Muscle flap was done using serratus anterior in order to cover the remaining lung surface, and Eloesser procedure was performed to drain the dead space (Figure 6). The mean hospital stay was 8.2 (range, 2–34) days, and 3 patients were readmitted.
Table 1
| Variables | Simple PA (N=59) | Complex PA (N=17) | P value |
|---|---|---|---|
| Age, mean, years | 53.9 | 56.1 | 0.570 |
| Gender, male, n (%) | 26 (44.1) | 9 (52.9) | 0.518 |
| Hemoptysis, n (%) | 42 (71.2) | 16 (94.1) | 0.120 |
| Tuberculosis, n (%) | 18 (30.5) | 11 (64.7) | 0.038 |
| BAE, n (%) | 11 (18.6) | 7 (41.2) | 0.054 |
| Location, upper, n (%) | 38 (64.4) | 10 (58.8) | 0.448 |
| Extent, lobectomy, n (%) | 34 (57.6) | 15 (88.2) | 0.001 |
| VATS, n (%) | 36 (61.0) | 2 (11.8) | 0.001 |
| Size of lesion, mean, cm | 3.06 | 3.66 | 0.200 |
| Stump coverage, n (%) | 14 (16.9) | 6 (35.3) | 0.360 |
| Spillage, n (%) | 2 (3.4) | 2 (11.8) | 0.180 |
| Complication, n (%) | 6 (10.2) | 7 (41.2) | 0.003 |
| Hospital stay, mean, d | 7.2 | 11.8 | 0.008 |
PA, pulmonary aspergilloma; BAE, bronchial artery embolization; VATS, video-assisted thoracic surgery.
Discussion
Despite recent advancements in minimal invasive surgery, VATS for PA remains challenging due to tight adhesion and massive bleeding. Decisions to perform surgery must adhere to surgical indications, such as recurrent hemoptysis, persistent sputum, and cough, that severely impede the quality of life. Spillage of aspergilloma in the surrounding healthy lung can disseminate the infection and lead to severe pneumonia, empyema, sepsis, and respiratory failure. Contamination of the healthy lung under anesthesia and during surgical manipulation was a concern. To minimize spillage of pus from the cavity to the contralateral lung and other ipsilateral lungs, one lung ventilation using double lumen endotracheal tube and separation of the affected lobe using bronchial block are crucial steps. Although a DLT would have protected the contralateral lung, the ipsilateral lobes need to be isolated against contamination. Concerning this, Sharma et al. suggested that once its position is confirmed, a Fogarty catheter should be introduced through the tracheal lumen to isolate the RUL bronchus from the rest of the right lung (4). Another method is dividing the bronchus of the affected lobe before handling and dissecting the adhesion; however, in lobes with inflammatory disease, locating the bronchus via hilar dissection is challenging. In the case of adhesion combined with pulmonary tuberculosis, there seemed to be tight adhesion especially around the cavity. Dissection of the apex requires great caution to avoid vascular injury. Extrapleural dissection is one of the methods used for reducing the risk of disruption of the aspergilloma in the pleural space with dissemination of the fungal infection, and it also minimizes the risk of intraoperative and early postoperative major bleeding. Lung parenchyma disruption is one of the reasons why there is frequent occurrence of post lobectomy empyema. A thoracoscopic guide is very useful during adhesiolysis, as it magnifies the target points, reducing the chances of intraoperative bleeding and vascular injury. The apical part is very hard to dissect via open thoracotomy because the intercostal space is very narrow in complicated aspergilloma. We also found that using an energy device may reduce bleeding during adhesiolysis.
The obliteration of residual pleural cavity reduces the risk of pleural empyema from incomplete filling of the pleural space by the remaining lobes (5). Thoracoplasty and muscle flap are the most common methods used to treat these dead space problems. Thoracoplasty is not frequently used nowadays; however, when the dead space is located on the upper lung fields, it may be very effective, especially in patients with very low muscle volume. A muscle flap is used to cover the injured lung parenchyma or to fill-up the infected space. Usually, serratus anterior or latissimus dorsi is used. The omental flap has the potential to be used in a wide range of thoracic surgeries, including chest wall reconstructions. Under normal circumstances, the omentum serves primarily as a protective tissue in the abdomen, although it has been shown to exhibit a variety of other functions, including cell transport, angiogenesis, absorption of bodily fluids, and immune regulation. Therefore, it can be useful to fill up the whole cavity after pneumonectomy or post-pneumonectomy empyema combined with bronchopleural fistula. The newly introduced vacuum-assisted closure therapy after open window thoracostomy maybe useful in postpneumonectomy empyema, however it requires careful evaluation and solid experience, because it can trigger clinically significant complications.
The size of aspergilloma and presence of underlying pulmonary disease should be considered when planning and determining the extent of surgery. The main goal is to resect the mycotic cavity, including in-going pulmonary vessels (5). At the same time, because most patients have decreased pulmonary reserve, the parenchymal resection should be limited as much as possible to avoid impairing lung function. The standard surgical approach includes anatomic pulmonary resections, such as segmentectomy or lobectomy; wedge resection should be reserved for small lesions. For complex PA, bilobectomy or pneumonectomy is sometimes inevitably performed for resection of all diseased lungs.
Coverage of a bronchial stump with viable tissue (pericardial, omental, and muscle flap) decreases the risk of bronchopleural fistula from bronchial deficiency due to chronic inflammation, atrophy, and calcifications. However, in simple aspergilloma, bronchial margin is usually clean; hence, there is no need to reinforce the bronchial stump.
For surgical approach, young patients or high-risk patients with localized PA, no severe pulmonary or pleural scarring, and absence of lymph nodes near pulmonary vessels, are eligible for VATS (6). The resection of infected or purulent lesions through minimal access may lead to contamination of the pleural cavity and wounds, resulting in empyema and wound infection. It may be important to emphasize that excessive dissection of the peribronchial tissue should be avoided to preserve perfusion. Another important surgical aspect to emphasize is that bronchoscopy must be performed as a preoperative routine to confirm that there is no engorgement or edema in the tunica mucosa (6).
In summary, surgery is only an effective treatment method for symptomatic patients with PA. In the case of simple PA, the operative risk is low, and VATS may be possible. However, in patients who have recurrent hemoptysis and complex PA, the operative risk may be high with increased risk of postoperative complications; such patients should avoid extensive surgical intervention.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kamran Ali) for the series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.03.04). The series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by IRB institutional ethics board (NO. B-1903-529-101) and given waiver of consent from patients because Video clip and Figure has no identification information.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Akbari JG, Varma PK, Neema PK, et al. Clinical profile and surgical outcome for pulmonary aspergilloma: a single center experience. Ann Thorac Surg 2005;80:1067-72. [Crossref] [PubMed]
- Weber A, Stammberger U, Inci I, et al. Thoracoscopic lobectomy for benign disease--a single centre study on 64 cases. Eur J Cardiothorac Surg 2001;20:443-8. [Crossref] [PubMed]
- Cho S. Right upper lobectomy by VATS was performed in patients with PA. Asvide 2019;6:059. Available online: http://www.asvide.com/article/view/30408
- Sharma A, Sinha S, Khanna S, et al. A novel technique to prevent endobronchial spillage during video assisted thoracoscopic lobectomy. Ann Card Anaesth 2014;17:164-6. [Crossref] [PubMed]
- Passera E, Rizzi A, Robustellini M, et al. Pulmonary aspergilloma: clinical aspects and surgical treatment outcome. Thorac Surg Clin 2012;22:345-61. [Crossref] [PubMed]
- Chen QK, Chen C, Chen XF, et al. Video-assisted thoracic surgery for pulmonary aspergilloma: a safe and effective procedure. Ann Thorac Surg 2014;97:218-23. [Crossref] [PubMed]
(English Language Editor: John Ayric Gray, AME Publishing Company)
Cite this article as: Cho S. Special issue on inflammatory thoracic disease: surgical experience of pulmonary aspergilloma. J Vis Surg 2019;5:25.