Minimally invasive resection of duodenal tumors
Introduction
Duodenal neoplasms are rare entities accounting for less than 1% of all gastrointestinal tumors characterized by their location in the duodenum and proximity to the ampulla. Truly benign duodenal tumors represent only 25% of all duodenal masses (1). Current treatment options include open surgical techniques (duodenal sleeve resection or transduodenal ampullary resection), laparoscopic-assisted surgery, and endoscopic resection [endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD)] (2-4).
The morbidities associated with laparotomy are well-established and can be significant (5,6). Endoscopic therapies have proven reliable and safe when selected to treat small, anatomically favorable lesions with low risk of malignancy, though there is an increased risk of perforation, especially with ESD, and an increased likelihood of piecemeal retrieval of the lesion, particularly with EMR (3). In some series, the risk of perforation during ESD for nonampullary adenomas has been reported to be as high as 35.7% (7). EMR features a reduced perforation risk relative to ESD, ranging from 0 to 4.3% (8). The rate of clinically significant bleeding following endoscopic resection ranges from 5% to 8.8% (9). ESD, while relatively morbid, offers up to 98% successful en bloc resection for nonampullary lesions; with EMR, this drops to 78% (10).
It is thus that robotic-assisted surgery has arisen as a reliable minimally invasive approach which permits a more extensive resection than can be done endoscopically without incurring the morbidities of open surgery (2). Our intent is to provide a review of robot-assisted surgical techniques used in the management of duodenal tumors, subdivided by tumor location within the duodenum.
Whereas laparoscopy has gained limited traction in this field due to the technical demands of duodenal reconstruction, the robotic platform bridges the gap between open and endoscopic resections and provides greater latitude in terms of patient selection for minimally-invasive surgery (2). The robotic approach is an oncologically safe technique for resecting larger tumors that enables the most complex reconstructions while retaining the patient benefits of minimally-invasive surgery.
We describe the operative technique and provide video instruction regarding duodenal lesions across all five levels of technical complexity: lesions located in the duodenal bulb (Type A), first portion of the duodenum (D1; Type B); nonampullary region of D2 (Type C); ampullary or juxta-ampullary (Type D), and transverse duodenum (D3/D4) distal to the ampulla (Type E) (2). Primary closure or Roux-en-Y duodenojejunostomy can be used after all five techniques close the duodenum without tension.
Operative techniques
Suggested equipment
- 5-mm optical separator for peritoneal entry;
- 12-mm Versaport trocar for the robotic camera;
- 5-mm Maryland LigaSureTM energy device;
- 5-mm suction irrigator;
- Intraoperative ultrasound;
- Da Vinci Robotic Surgery System with fenestrated bipolar; Prograsp; cautery hook; and large needle drivers (robotic instruments).
Positioning and trocar placement
The following guidance applies to all five types of robotic duodenal surgery. The patient is positioned supine on a split-leg operating table. The right arm is padded and tucked to prevent collisions with the two right-sided camera arms. The left arm is extended at 90 degrees to afford anesthesia access. A nasogastric tube, urinary catheter, and other appropriate monitoring devices are placed.
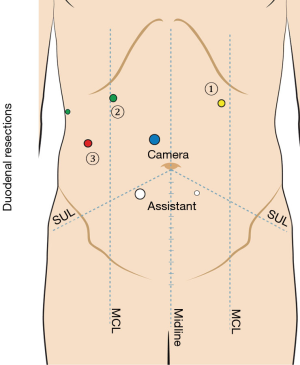
Access to the peritoneal cavity is obtained using a 5-mm optical separating trocar inserted two finger breadths below the mid-clavicular line in the left upper quadrant. Following this, the abdomen is insufflated to 15 mmHg with CO2. The falciform ligament is anchored to the abdominal wall to elevate segment IV, and a 5 mm trocar is routinely placed in the right anterior axillary line and used for a flexible liver retractor to elevate segment V from the hepatic flexure. Additional trocars are placed including one 12-mm camera trocar superior and to the right of the umbilicus; two 5-mm trocars in the right upper quadrant, one 12-mm assistant trocar in the right lower quadrant for passing needles, and a 5-mm trocar in the left lower quadrant for retraction and aspiration (Figure 1).
The duodenum and right colon are mobilized, and a Kocher maneuver is performed by means using the LigaSureTM. The duodenum is completely mobilized from the retroperitoneum with the distal landmark close to the ligament of Treitz, the proximal landmark at the foramen of Winslow, and extended medially to the SMA takeoff anterior to the descending aorta. Two sponges are used to rotate the duodenum 90 degrees to orient the lateral duodenum anteriorly. The Si robot is then docked over the patient’s head or right shoulder.
Transduodenal excision of lesion (Figure 2)

This technique applies to Type A and B resections located in the antimesenteric wall. After duodenal mobilization, intraoperative ultrasound is performed as required to localize the lesion relative to the proposed location of duodenal incision. A stay suture is placed cephalad to the longitudinal duodenotomy to maximize retraction, and cautery scissors are used to open the duodenum and circumferentially resect the lesion under visual guidance. The surgical margins may be evaluated by frozen section as needed, though is not done routinely as the macroscopic boundaries of most adenomas are clearly visible. The duodenotomy is repaired transversely in two layers taking care to imbricate the ends of the suture line to prevent a leak, using 4-0 V-Loc® suture in a Connell technique followed by serosal reapproximation with interrupted 3-0 silk Halstead sutures. Esophagogastroduodenoscopy is performed as needed to assess for leak and confirm luminal patency. A surgical drain is not routinely employed. A Roux-en-Y duodenojejunostomy may be employed to reconstruct the duodenal wall when tension threatens anastomotic integrity.
Segmental duodenal (sleeve) resection (Figure 3)

Duodenal lesions involving the medial wall and requiring segmental resection must be carefully localized to avoid disconnecting either the major or minor papilla from the GI tract. Dividing the duodenum in close proximity to the ampulla can also compromise biliary and pancreatic drainage with the expected results.
Preoperative upper endoscopy can be used to place an endoscopic clip on the lesion which is then visualized with intraoperative ultrasound, a useful method as distention of the duodenal lumen with fluid to improve delineation of tumor from ampulla is not practical intraoperatively. This technique is useful in Type A, B, and E resections when the lesion occupies a majority of the duodenal circumference, provided that there is sufficient space to spare the ampulla. Tattoos usually stain the entire region of the duodenum and are not sufficiently definitive marking for this purpose. Cannulating the common bile duct with a 5 French balloon Fogarty catheter through the cystic duct stump after cholecystectomy is the most direct method of establishing the location of the ampulla relative to medial lesions.
If the location of the ampulla is sufficiently distant from the lesion to clear the anticipated margin, a liner cutting stapler can be used to divide the proximal or distal duodenum as indicated. Given the sort distances involved, we usually prefer to divide the duodenum under direct vision with the cautery scissors to maintain a safe visual perimeter to the ampulla. The LigaSure® device is then used to divide the duodenal mesentery and small perforating branches emanating from the head of the pancreas. Suture ligation should be used liberally, as any degree of postoperative pancreatitis may cause sealed arterioles to bleed. Though not done routinely, frozen histologic sections can be done to confirm complete excision.
Continuity is restored with side-to-side or end-side duodenojejunostomy in two layers as described below. Following D2 resections, we prefer to bring the Roux limb through the bare area of the transverse colon to simplify alignment and visualization of the posterior row of alignment sutures.
Ampullectomy with ampullary reconstruction (Figure 4)

This technique is used to resect and reconstruct lesions of the ampulla or immediate juxta-ampullary duodenum (Type D). The ampulla is identified as presented above. A longitudinal duodenotomy is created using electrocautery scissors, and a 2-0 silk is placed in the medial wall of the duodenum proximal to the ampulla to facilitate exposure. Using cautery scissors, the mucosa is incised 5–10 mm away from visible adenomatous change is incised to expose the submucosal plane and carried circumferentially around the lesion. Carefully identifying the submucosal plane permits hemostasis to be maintained as feeding vessels cross it into the mucosa and can be controlled. Vigilance to entry into the pancreatic duct is high during this portion of the dissection, as, once missed, the small pancreatic duct can be quite difficult to identify subsequently. When encountered the pancreatic duct is cannulated with an ECP pancreatic duct stent of appropriate size. The pancreatic duct is usually seen at the 6 o’clock position after incising the bile duct. The bile duct is constantly in view due to the Fogarty catheter. The specimen is placed in an Endocatch® bag and retrieved through the right lower quadrant trocar. Frozen section analysis is not routinely performed for lesions which are completely grossly excised. The duodenal mucosa is re-approximated to the bile duct mucosa using 5-0 Vicryl interrupted sutures beginning at 12 o’clock and continuing in a clockwise fashion. Additional sutures are placed into the septum between the pancreatic duct and bile duct to ensure continued patency of both ducts. The duodenum is closed in a transverse fashion as presented. If desired, an omental patch may be placed over the duodenal closure. Drains are not routinely used.
Near-circumferential duodenectomy with Roux-en-Y duodenojejunostomy (Figure 5)

Excising lesions which occupy a significant portion of the duodenal circumference may create a large defect which cannot be closed primarily. Under these circumstances, the duodenectomy is reconstructed using a Roux-en-Y side-side duodenojejunostomy. During excision of the duodenal mass, a defect in the transverse mesocolon is created. The jejunum is divided at the point of maximum vascular mobility using an EndoGIA stapling device, and the mesentery is divided with Ligasure as the crossing arcades are observed to prevent ischemia. After the jejunojejunostomy is created and the mesenteric defect is closed, the Roux limb is passed cephalad through the mesocolic defect to lie adjacent to the duodenum. The duodenum is tacked to the jejunum with interrupted seromuscular stitches to maintain its orientation, and a side-side running anastomosis of 4-0 V-Lok is used to create a two-layer anastomosis to the retro-colic Roux limb. loop by means of a side-to-side duodenojejunostomy.
Post-operative management
Surgical drains are not routinely used. A nasogastric (NG) tube placed under direct vision intra-operatively is left to low continuous suction overnight. If the output is low, the tube is usually removed on post-operative day #1 followed by a trial of liquids. If the patient fails to tolerate a diet, a 24-hour trial of NG decompression is attempted to determine whether a high-protein liquid diet or total parenteral nutrition (TPN) will be required while awaiting resolution of edema. In this series, all patients were able to resume regular diet within six days post-operatively, and none required TPN supplementation.
Post-operative outcomes
This institutional case series of robot-assisted duodenal resection supports the safety and feasibility of these described surgical techniques for a variety of symptomatic benign and premalignant lesions. Sixteen patients have undergone robot-assisted transduodenal resections between June 2013 and November 2017. Surgical indications included tubulovillous adenomas [13], neuroendocrine tumors [2] and leiomyomas [1]. The tumors were located in the ampulla [7], duodenum [8] and the minor papilla [1] with a median tumor size of 2.85 cm (1.5–5.0 cm). Data is shown in Table 1. Two patients had undergone prior EMR and developed recurrence prior to robotic surgery. One of these patients underwent polypectomy two years prior to surgery at an outside hospital, during which polyp tissue could not be retrieved. The other of these patients underwent two EMRs five years prior to surgery; the first of these was a piecemeal polypectomy, followed one year later by endoscopic polypectomy of the residual polyp. This patient finally underwent surgery after developing a bleeding ulcer at the polypectomy site. Operative time ranged from 214 to 393 minutes with an estimated average blood loss of 50 mL. There were no conversions to open surgery or intra-operative blood transfusions. Following operation, the average length of stay was 7 days with no duodenal leaks, pancreatic fistulas, re-operations on index hospitalization, disease recurrences, or death.
Table 1
| Case details | Ampullary involvement, n=9 | No ampullary involvement, n=7 |
|---|---|---|
| Tumor size (median, IQR) | 2 [1.2–3.85] cm | 2.7 [2.1–3.2] cm |
| OR time (median, IQR) | 320 [287–372] min | 271 [214–370] min |
| Estimated blood loss (mean, IQR) | 67 [23–100] mL | 38 [10–50] mL |
| Length of stay (median, IQR) | 8 [6–8] days | 6 [4–8] days |
Discussion
Historically, pancreaticoduodenectomy was the operation of choice to clear potential invasive carcinoma; however, open trans-duodenal resection has provided surgeons with a viable surgical alternative for the management of benign and premalignant duodenal lesions. In the largest series comparing open ampullectomy versus pancreaticoduodenectomy, for example, pancreaticoduodenectomy patients demonstrate higher rates of complications including delayed gastric emptying (16% versus 0%) and pancreatic leak (20.7% versus 0%) (15).
While open duodenal surgery is now considered standard of care management for primary duodenal and ampullary neoplasms at most centers, the morbidity of this approach remains substantial and confers relatively higher rates of lesion recurrence and the need for ongoing endoscopic surveillance (5). In a prospective study by de Castro et al., both open local resection and pancreaticoduodenectomy for benign duodenal neoplasms yielded 100% R0 resection rate. Local recurrence developed in 1 of 12 patients who underwent local resection compared to zero of 7 patients after pancreaticoduodenectomy (16). While less morbid than pancreaticoduodenectomy, open transduodenal surgery still causes considerable morbidity including delayed gastric emptying, wound infection and pancreatitis in nearly 50% of patients (6,17,18).
Endoscopic alternatives developed in response to relatively high morbidity after open surgery for primary duodenal and ampullary lesions. The two best-established techniques are endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). Both techniques have risks including post-procedural bleeding, perforation, incomplete lesion removal, and a higher risk of lesion recurrence (2,19). As a result, routine post-procedure surveillance is required after ESD and EMR. Further, endoscopic removal cannot safely be endorsed for routine management and should be reserved for small benign lesions spanning at most one third of the luminal circumference (19).
The robotic approach provides several advantages specific to duodenal and ampullary lesions. Ampullectomy and reconstruction of the biliary and pancreatic ductal orifices often necessitate extremes of instrument articulation. The robotic platform affords a much greater degree of wrist articulation relative to laparoscopy, as well as superior three-dimensional views and computer-based compensation for operator tremor. Ultimately, the robotic platform offers a minimally-invasive option for transduodenal and ampullary surgery which affords the flexibility and diminished risk of unrecognized bowel injury relative to laparoscopy (20). While robotic duodenal resection with or without ampullary reconstruction is not yet routine in clinical practice, our experience suggests that judicious patient selection and careful technique may permit successful lesion removal without recurrence in up to 100% of patients.
Tips, tricks and pitfalls
- Preoperative cross-sectional imaging and endoscopic intra-operative ultrasound (IOUS) should be used to define the expected anatomic boundaries, exclude unrecognized invasion, and establish proximity to the ampulla of Vader.
- Intraoperative ultrasound should be the surgeon’s GPS system to minimize the risk of margin contamination or ampullary injury.
- Care must be exercised during duodenal closure to prevent inadvertent gathering of redundant tissue known as a “dog-ear deformity”, as this will necessitate imbrication and may cause significant luminal narrowing or tension.
- The surgical margin should be carefully scrutinized to be ensure tumor clearance. This often requires frozen section evaluation or specimen ultrasound.
Conclusions
Robotic duodenal surgery offers a feasible, minimally-invasive strategy that reduces operative time and length of stay with equivalent pathological outcomes to open surgery. This is particularly advantageous in light of the limitations of endoscopic resection of these lesions. While larger studies are still needed to confirm these findings, robotic duodenal surgery with and without ampullary reconstruction represents an emerging minimally-invasive platform with demonstrated efficacy in the management of benign and pre-malignant duodenal lesions.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.02.01). AJM serves as an unpaid editorial board member of Journal of Visualized Surgery from Dec 2017 to Nov 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yan JQ, Peng CH, Yang WP, et al. Surgical management of benign duodenal tumours. ANZ J Surg 2010;80:526-30. [Crossref] [PubMed]
- Downs-Canner S, Vliet WJ, Thoolen SJ, et al. Robotic Surgery for Benign Duodenal Tumors. J Gastrointest Surg 2015;19:306-12. [Crossref] [PubMed]
- Shibagaki K, Ishimura N, Kinoshita Y. Endoscopic submucosal dissection for duodenal tumors. Ann Transl Med 2017;5:188. [Crossref] [PubMed]
- Stauffer JA, Raimondo M, Woodward TA, et al. Laparoscopic partial sleeve duodenectomy (PSD) for nonampullary duodenal neoplasms: avoiding a whipple by separating the duodenum from the pancreatic head. Pancreas 2013;42:461-6. [Crossref] [PubMed]
- Stauffer JA, Adkisson CD, Riegert-Johnson DL, et al. Pancreas-sparing total duodenectomy for ampullary duodenal neoplasms. World J Surg 2012;36:2461-72. [Crossref] [PubMed]
- Di Saverio S, Segalini E, Birindelli A, et al. Pancreas-sparing, ampulla-preserving duodenectomy for major duodenal (D1–D2) perforations. Br J Surg 2018;105:1487-92. [Crossref] [PubMed]
- Jung JH, Choi KD, Ahn JY, et al. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy 2013;45:133-5. [Crossref] [PubMed]
- Sohn JW, Jeon SW, Cho CM, et al. Endoscopic resection of duodenal neoplasms: a single-center study. Surg Endosc 2010;24:3195-200. [Crossref] [PubMed]
- Lepilliez V, Chemaly M, Ponchon T, et al. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy 2008;40:806-10. [Crossref] [PubMed]
- Hoteya S, Furuhata T, Takahito T, et al. Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Non-Ampullary Superficial Duodenal Tumor. Digestion 2017;95:36-42. [Crossref] [PubMed]
- Cervoni GE, Singer T, Decicco C, et al. Transduodenal excision of lesion. Asvide 2019;6:052. Available online: http://www.asvide.com/article/view/30175
- Cervoni GE, Singer T, Decicco C, et al. Segmental duodenal (sleeve) resection. Asvide 2019;6:053. Available online: http://www.asvide.com/article/view/30176
- Cervoni GE, Singer T, Decicco C, et al. Ampullectomy with ampullary reconstruction. Asvide 2019;6:054. Available online: http://www.asvide.com/article/view/30178
- Cervoni GE, Singer T, Decicco C, et al. Near-circumferential duodenectomy with RNY DJ. Asvide 2019;6:055. Available online: http://www.asvide.com/article/view/30180
- Winter JM, Cameron JL, Olino K, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg 2010;14:379-87. [Crossref] [PubMed]
- de Castro SM, van Heek NT, Kuhlmann KF, et al. Surgical management of neoplasms of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery 2004;136:994-1002. [Crossref] [PubMed]
- Posner S, Colletti L, Knol J, et al. Safety and long-term efficacy of transduodenal excision for tumors of the ampulla of Vater. Surgery 2000;128:694-701. [Crossref] [PubMed]
- Golhar A, Mangla V, Mehrotra S, et al. Limited distal duodenal resection: Surgical approach and outcomes. A case series. Ann Med Surg (Lond) 2018;30:36-41. [Crossref] [PubMed]
- Adler DG, Qureshi W, Davila R, et al. The Role of Endoscopy in Ampullary and Duodenal Adenomas. Gastrointest Endosc 2006;64:849-54. [Crossref] [PubMed]
- Testini M, Piccinni G, Lissidini G, et al. Management of Descending Duodenal Injuries Secondary to Laparoscopic Cholecystectomy. Dig Surg 2008;25:12-5. [Crossref] [PubMed]
Cite this article as: Cervoni GE, Singer T, Decicco C, Critchlow JF, Kent TS, Moser AJ. Minimally invasive resection of duodenal tumors. J Vis Surg 2019;5:20.