Management of hemoptysis in inflammatory chest diseases
Introduction
Hemoptysis is the expectoration of blood or blood-stained sputum originating from the lung or the bronchus. In general, patients with hemoptysis have self-limited type of bleeding, and <5% of the patients are hospitalized with massive hemoptysis that definitely requires an urgent intervention. The expectoration of >100 mL of blood over a period of 24 hours could be described as massive hemoptysis, although the volumetric description may be as high as 1 liter as reported in the previous decade (1). Other descriptions of hemoptysis may include expectoration of a large amount of blood that endangers the life of a patient or creates a clinical scenario with morbid outcome.
For the management of hemoptysis, it is necessary to identify the cause. In this article, we shall discuss about the expectoration of blood other than upper airway bleeding, gastrointestinal bleeding, and neoplasms. The reasons for this type of expectoration are bronchitis, bronchiolitis, airway traumas, foreign body aspirations, lung abscess, pneumonia, tuberculosis, fungus ball, Goodpasture syndrome, idiopathic pulmonary hemosiderosis, Wegener’s granulomatosis, lupus pneumonitis, lung contusion, arteriovenous malformation, pulmonary embolism, elevated pulmonary venous pressure, pulmonary endometriosis, and systemic coagulopathy (Table 1).
Table 1
| Infection diseases |
| Tuberculosis |
| Bronchitis |
| Pneumonia |
| Lung abscess |
| Bronchiectasis |
| Aspergilloma |
| Cystic fibrosis |
| Cysts hydatic |
| Cardiac diseases |
| Mitral stenosis |
| Left lung failure |
| Vascular diseases |
| Pulmonary emboli/infarct |
| Bronchovascular fistula |
| Arteriovenous malformation |
| Malignancies |
| Lung cancer |
| Carcinoid tumor |
| Metastatic tumors |
| Trauma |
| Thorax trauma |
| Tracheovascular fistula |
| Bronchial rupture |
| Iatrogenic |
| Bronchoscopy /transthoracic biopsy |
| Swan-Ganz catheter |
| Anticoagulant and fibrinolytic treatment |
| Syndromes of alveolar hemorrhage |
| Vasculitis (Behçet’s diseases, Wegener) |
| Connective tissue diseases |
| Goodpasture syndrome |
| Hematologic diseases |
| DIC, haemophilia, thrombocytopenia |
| Others |
| Catamenial hemoptysis, pneumoconiosis |
| Idiopathic |
DIC, disseminated intravascular coagulation.
Bronchiectasis and chronic bronchitis are the most common causes in the non-malignant hemoptysis category (2).
When a patient is referred with hemoptysis, it is necessary to confirm it before performing an invasive intervention or surgery. Bleeding in the oral cavity and the nasal fossa, basically an upper airway bleeding or a gastrointestinal bleeding, should be differentiated from hemoptysis through a detailed physical examination and careful history-taking. In addition to physical examination, rhinolaryngoscopy, endoscopy, or bronchoscopy could be required in these situations. For this reason, patients with hemoptysis should be directed to a hospital that has the abovementioned facilities.
Although anterior-posterior and lateral X-ray scans are recommended as initial imaging methods, we always perform an emergency contrast-enhanced chest computed tomography (CT) for patients with hemoptysis. Conventional CT scan correctly localizes the sources of bleeding in 88.5% of the cases as reported by Haponik et al. (3). Most often, the lesions are bronchiectasis, infections (pneumonia, tuberculosis, fungal), or lung tumors. However, conventional techniques may be insufficient in some cases, such as in patients with arteriovenous malformations (4). In addition, this multidetector CT could substitute bronchoscopy as the first-line method for the evaluation of patients with hemoptysis (5). In case there are more suspicious areas in the radiological examination, fiberoptic bronchoscopy can be used as a guide to identify the focus of hemoptysis.
Nonmassive hemoptysis can be managed by conservative treatment options such as treatment of the infection or the inflammatory disease according to the underlying pathology, and the outcome may be satisfactory in most of the cases (6). Stabilization of coagulation by medical measures is another method to stop the bleeding. Antifibrinolytic treatment with tranexamic acid has been recommended to control hemoptysis in patients with the following etiologies: pneumonia, vasculitis (Behçet syndrome, Goodpasture syndrome), bronchiectasis, and cystic fibrosis (6).
In this article, we would like to share our experience of our approach toward three different patients with hemoptysis. The first case was a patient with a first-time experience of a massive hemoptysis who underwent an emergency video-assisted thoracoscopic surgery (VATS) resection within 1 hour after her admission. The second case was an elective candidate of VATS resection. The third patient was managed with bronchial embolization.
Case presentation
Case 1: hydatid cysts
There is no description about parasitic causes of hemoptysis such as Echinococcus granulosus as one of the common reasons for hemoptysis; thus, its incidence can increase according to endemicity of the geographical area (7). Treatment options for pulmonary hydatid cysts include pharmacotherapy and/or surgery. Although surgery is the preferred method, pharmacotherapy (benzimidazoles) may also be useful in selected patients. Indications for medical therapy include smaller cysts, and patients with contraindication for surgery could be described as those with poor surgical risk, multiorgan disease, multiple cysts, recurrent cysts, and intraoperative spillage of hydatid fluid (8).
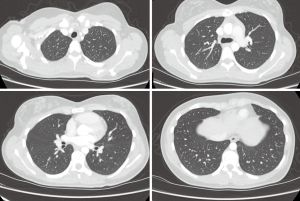
This case was a 47-year-old female patient who was hospitalized with a first-time experience of a massive hemoptysis, and the volume of bleeding was approximately 0.5 liter. An emergency chest CT demonstrated lesions compatible with lung pathology of cystic nature (Figure 1).
Fiberoptic bronchoscopy was performed in the operating room under general anesthesia, and we observed that fresh coagulum was obstructing the right lower lobe orifice. We did not want to draw the coagulum and cause more bleeding and hence intubated the patient with a left endobronchial intubation tube and started unilateral lung isolation and ventilation. Videothoracoscopic surgery was performed via three ports. The artery and vein of the lower lobe and the bronchus were stapled, and lower lobectomy was performed. Pathology revealed a perforated hydatid cyst with erosion of pulmonary artery on the interlobar level. The duration of stay in the hospital was 4 days, and the patient was discharged without complications. She was prescribed albendazole treatment. During her 3-year follow-up examinations, she did not develop any cysts.
Case 2: bronchiectasis
The incidence and treatment strategy of bronchiectasis have been updated in recent years according to the progress in the development of antibiotics. Medical therapy could be insufficient, and recurrent infections and massive hemoptysis could occur. Surgery could be a good option in localized disease by resection of affected areas. If the bronchiectasis is diffuse and homogeneous, limiting the patients’ daily life bilateral lung transplantation could be considered (9).
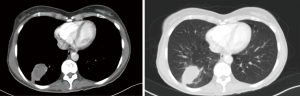
A 41-year-old male patient was referred to our department from the chest disease department with severe hemoptysis due to underlying bronchiectasis. Hemoptysis continued despite bronchial artery embolization, which was applied in a different center. Multiple bronchiectatic areas were detected in the thorax CT, especially in the left lower lobe, the lingula, and the right middle lobe (Figure 2).
He underwent medical therapy (antibiotherapy and antifibrinolytic treatment) at the chest disease clinic. Because he had massive hemoptysis and his clinical findings began to deteriorate, such as decreasing saturation and blood pressure, we decided to perform an emergency operation. First, fiberoptic bronchoscopy was performed in the operating room, followed by intubation under general anesthesia. The right bronchial system and the left upper lobe were completely clean, but a coagulum was detected in the bronchus of the common basal segment. There was an oozing type of bleeding under the coagulum, which was partially obstructing the left lower lobe common basal segment. The patient was intubated with a left endobronchial tube, and the lung was isolated.
He underwent biportal videothoracoscopic surgery in the same setting. The bronchiectasis was especially localized to the common basal segment of the lower lobe and the lingula of the upper lobe; common basal segmentectomy and lingulectomy were performed to save the healthy residual parenchyma.
The patient was discharged from the hospital on the fourth postoperative day after the surgery without any complication.
Case 3
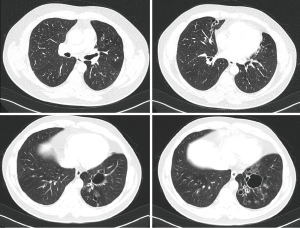
A 22-year-old female patient was referred to us because of recurrent moderate hemoptysis in the past 1 year, especially localized menstrual periods. She was examined and treated by the gynecology department for a suspicion of pulmonary endometriosis. When she was evaluated by the chest disease department, no pathology was found on the CT (Figure 3). Fiberoptic bronchoscopy that was performed during a period of hemoptysis did not reveal any active hemorrhage.
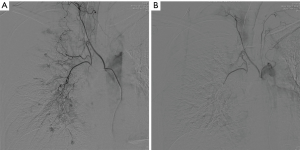
Because moderate and recurrent hemoptysis periods continued, she applied to the chest surgery clinic. Echocardiography and blood examinations for the bleeding profile were normal. She then underwent bronchial angiography. There were hypervascularization areas in both lungs, and the largest bronchial artery was located in the left lower lobe. An arteriovenous fistula was also present in the right upper lobe. Embolization was performed to both bronchial arteries (Figure 4). After the embolization, she was referred to the connective tissue disease department for the examination of vasculitis. She developed no hemoptysis since the procedure.
Conclusions
Non-malignant and inflammatory causes of hemoptysis are more common in this part of the world. However, modern world surgeons should be aware of major hemoptysis causing infection and inflammatory pathologies due to increasing immigration from poor countries to socioeconomically developed countries. Treatment strategies can change according to the etiology, and the primary types of treatments include medical management, embolization, and surgery.
Yun et al. have shared their experiences in patients who had hemoptysis with benign lung diseases. They preferred sublobar resections. In addition, they reported that videothoracoscopic surgery and elective (planned) operations have better outcomes (10). However, adhesions and anthracotic lymph nodes present difficulties in these types of operations, although VATS can be safely applied by experienced surgeons (10). Baysungur et al. declared that VATS resections are limited for infectious diseases, especially bronchiectasis, because of dense pleural adhesions, multiple lymph nodes, and spiral bronchial arteries. The rate of conversion to open surgery was 6.8% in their bronchiectasis series (11). Wang et al. analyzed 35 patients with sequestration and reported that all of them underwent resection via VATS with shorter operation duration and less bleeding (12).
We would like to suggest sublobar resections, namely, segmentectomy operations, for patients with bronchiectasis. We believe that VATS is an optimum intervention in experienced hands. Although we perform robotic lung resections, we would not consider this approach in a patient with major hemoptysis. However, this option may be considered under elective circumstances.
Robot-assisted thoracoscopic surgery has superiorities such as endowrist and 3D visualization that allow safe dissection of adhesions, lymph nodes, and/or around the bronchovascular structures (13). We believe that performing the robot-assisted method has some advantages for patients who are well prepared and operated on under elective circumstances. Certainly, the cost and the longer duration of docking time may be a disadvantage. We believe that an experienced surgeon can overcome the disadvantage of long docking time as reported by Melfi et al., who declared that their four-patient series with sequestration underwent robotic resections in a mean operative time of 130 min (range, 100–150 min) (14).
In conclusion, various reasons can cause non-malignant hemoptysis, and the surgical strategy can change according to the etiology. Sublobar resections and minimally invasive surgery have become preferable in recent years.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kamran Ali) for the series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.01.12). The series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” was commissioned by the editorial office without any funding or sponsorship. AT serves as an unpaid editorial board member of Journal of Visualized Surgery from Jun 2017 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salajka F. The causes of massive hemoptysis. Monaldi Arch Chest Dis 2001;56:390-3. [PubMed]
- Cordovilla R, Bollo de Miguel E, Nuñez Ares A, et al. Diagnosis and Treatment of Hemoptysis. Arch Bronconeumol 2016;52:368-77. [PubMed]
- Haponik EF, Britt EJ, Smith PL, et al. Computed chest tomography in the evaluation of hemoptysis. Impact on diagnosis and treatment. Chest 1987;91:80-5. [Crossref] [PubMed]
- Mall S, Sharma RK, Prajapat D, et al. Hemoptysis: Beyond routine chest computed tomography and bronchoscopy. Lung India 2017;34:368-71. [Crossref] [PubMed]
- Davoodi M, Kordi M, Gharibvand MM, et al. Hemoptysis: comparison of diagnostic accuracy of multi detector CT scan and bronchoscopy. Glob J Health Sci 2015;7:373-7. [Crossref] [PubMed]
- Earwood JS, Thompson TD. Hemoptysis: evaluation and management. Am Fam Physician 2015;91:243-9. [PubMed]
- Toker A, Tanju S, Bayrak Y, et al. Life-threatening hemoptysis in a child: the only symptom. Ann Thorac Surg 2004;77:336-8. [Crossref] [PubMed]
- Sarkar M, Pathania R, Jhobta A, et al. Cystic pulmonary hydatidosis. Lung India 2016;33:179-91. [Crossref] [PubMed]
- De Dominicis F, Andréjak C, Monconduit J, et al. Surgery for bronchiectasis. Rev Pneumol Clin 2012;68:91-100. [Crossref] [PubMed]
- Yun JS, Song SY, Na KJ, et al. Surgery for hemoptysis in patients with benign lung disease. J Thorac Dis 2018;10:3532-8. [Crossref] [PubMed]
- Baysungur V, Dogruyol T, Ocakcioglu I, et al. The Feasibility of Thoracoscopic Resection in Bronchiectasis. Surg Laparosc Endosc Percutan Tech 2017;27:194-6. [Crossref] [PubMed]
- Wang S, Li Y, Wang J. Video-Assisted Thoracoscopic Surgery for Pulmonary Sequestrations: Series of 35 Consecutive Patients in a Single Center. Thorac Cardiovasc Surg 2019;67:73-8. [Crossref] [PubMed]
- Khan AZ, Ali K, Khandelwal S, et al. Robotic assisted thoracoscopic right upper lobectomy for post tuberculosis aspergilloma. J Vis Surg 2016;2:51. [Crossref] [PubMed]
- Melfi FM, Viti A, Davini F, et al. Robot-assisted resection of pulmonary sequestrations. Eur J Cardiothorac Surg 2011;40:1025-6. [PubMed]
Cite this article as: Cosgun T, Toker A. Management of hemoptysis in inflammatory chest diseases. J Vis Surg 2019;5:19.