Robotic-assisted thoracic surgery in Brazil, a review of the literature and our current experience
Introduction
Minimally invasive surgery (MIS) is widely regarded as the standard of care in the treatment of initial lung cancer and mediastinal malignancies. Minimally invasive lobectomy using a video assisted approach was first reported 25 years ago (1). The evidence available comparing video-assisted thoracic surgery (VATS) to open thoracotomy suggests reduced morbidity, length of stay and postoperative pain favoring the minimally invasive approach with no difference regarding oncologic outcomes (2-7). Due to these results VATS is strongly recommended by guidelines as the first option in the surgical treatment of lung cancer and other thoracic malignancies (8,9). Paradoxically, implementation of VATS has been slow and even after decades of experience with this method open thoracotomy has remained the most common approach in the surgical treatment of thoracic diseases (10,11). Bi-dimensional vision and limited instrument maneuverability may result in imprecise dissections and a difficult learning curve which in part explains the stagnation of VATS worldwide
Robotic-assisted thoracic surgery (RATS) is another form of thoracic MIS and solves some shortcomings of VATS. It offers 3D high definition viewing and lets the surgeon control the camera at will. Moreover, the robotic platform has articulated instruments (EndoWrist®) and tremor filtration, allowing for a more accurate and safer dissection. Several studies confirmed that oncologic outcomes are equivalent when comparing RATS to VATS lobectomy (12-16). Regarding intra and post-operative outcomes results are conflicting. Even though some studies showed no difference between RATS and VATS, others found that RATS was associated with lower conversion rates, less overall postoperative complications, and shorter hospital stay (10,17-20).
The main setback of RATS is its higher costs, directly associated to the large capital investment to acquire the robotic platform. In spite of the costs and lack of randomized evidence, from 2009 to 2013, RATS raised from 1% to 11% of all lobectomies performed at non-academic hospitals in the United States and more recent data suggests that this number is still increasing reaching over 17% (20,21). Due to the technical improvements and the potential postoperative benefits offered by robotics, thoracic surgeons followed a trend that is present in other surgical specialties worldwide.
By the end of 2017 the installed base of da Vinci® systems in clinical use reached 4,409 which represents a growth of 13% in comparison to 2016 and the number of procedures is expected to be close to a million a year by the end of 2018 (22). Well established in fields like urology and gynecology, robotic surgery has become ever so popular, with the technology reaching developing countries such as Brazil. Currently there are 41 da Vinci systems in Brazil. Although distributed among all five regions of the country, the states of São Paulo and Rio de Janeiro concentrate almost 75% of the nation’s surgical robots, indicating a potential for growth of the platform in other areas.
Despite the later onset of robotic surgery in our country, we believe that surgical care in Brazil is following the same trend as Europe and North America and RATS is becoming increasingly more available for the Brazilian thoracic surgeon. Here we present a review of the literature published in the country regarding this subject and describe our personal experience with the method.
Review of Brazilian literature
The Brazilian literature on this subject is extremely scarce. Very few scientific papers have been written. In fact, there are only three published articles and one poster presented in an international conference. The first study is a prospective randomized trial including 38 patients published in 2008. The objective was to compare surgical safety and efficacy between robotic and human camera control in video-assisted thoracic sympathectomy for hyperhidrosis. Camera holder robotic system AESOP® (Automated Endoscopic System for Optimal Positioning, Computer Motion Inc., USA) was used in half of the cases. There was no difference between groups regarding surgical accidents, number of involuntary movements, pain, aesthetical results, general satisfaction, number of lens cleaning, anhidrosis, length of hospitalization, and compensatory hyperhidrosis. The number of contacts of the laparoscopic lens on mediastinal structures was lower in the robotic group (P<0.001), however with higher surgical and camera use times (12.89±3.38 vs. 9.89±2.96 min and 7.55±2.97 vs. 4.59±1.99 min, respectively). The study concluded that camera holding by a robotic arm is as safe but less efficient than human control (23). This study, though representing the first published experience with robotics in Brazilian thoracic surgery, serves more as a historical note as the AESOP system has been discontinued since then and the da Vinci surgical system is the only FDA approved robotic surgical system on the market currently.
The second paper, published in 2011, is a case report of a patient with Myasthenia Gravis who underwent robotic thymectomy (24). In this case the da Vinci surgical robotic system was already used. The surgery and the postoperative period were uneventful. The total operative time was 120 min. The chest tube was removed 48 hours after surgery, and the patient was discharged 72 hours after surgery. The authors concluded that the robotic approach was safe and allowed a radical resection of all thymic tissue and mediastinal fat.
General thoracic and cardiothoracic surgeons of a single Brazilian private institution presented their experience at the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) conference in 2013 (25). From 2010 to 2012 they performed 27 robotic surgeries: four lobectomies, seven mediastinal tumor resections (six anterior and one posterior) and 16 cardiac surgeries. There were no operative deaths and no conversions to thoracotomy.
The last paper, published in 2016, described the implementation of a robotic thoracic program at a public tertiary teaching hospital (26). It was a planned interim analysis of a randomized clinical trial aimed at comparing VATS and RATS in terms of the results obtained after pulmonary lobectomy. Ten patients were included, all of them presented with peripheral tumors. Right upper lobectomy was performed in four patients, right lower lobectomy in four, and left upper lobectomy in two. Surgical time varied considerably (mean 277.3 min, range, 135–435 min). There were no intraoperative complications or conversion to open or VATS. Only the first patient required postoperative transfer to the ICU. There were no deaths or readmissions within the first 30 days after discharge. The only postoperative complication was chest pain, which occurred in two patients. Pathological examination revealed complete tumor resection in all cases. This study was published by our group and marked our initial experience with RATS starting in early 2015. We concluded that, in the presence of an institutional program and proper training of a multidisciplinary team, robotic thoracic surgery could be safely implemented with satisfactory results from the very beginning. Final results of this trial will be published soon.
Personal experience
The initial outcomes of the aforementioned study were very encouraging and prompted us to further develop our robotic surgery experience. This coincided with the expansion of robotic surgery in Brazil and the acquisition of many robotic platforms, mainly by private hospitals, around the country. Since then we have performed more than 200 operations in various centers with preliminary results that we will present in more detail.
Pulmonary resections
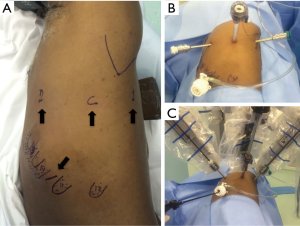
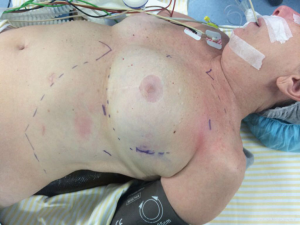
For pulmonary resections, we use a slightly modified version of the robotic lobectomy technique originally described by Dylewski et al. (27). Patients are placed in a lateral decubitus position. A total of four ports are used: three for the robotic arms (surgical scope, thoracic grasper and bipolar Maryland) and one, on the 10th intercostal space, used by the assistant surgeon for exposure, aspiration, stapling, introduction/removal of materials (such as gauze), and removal of specimens for pathological examination (Figure 1). We also use, through this lower port, CO2 insufflation which we believe has many advantages such as increasing the workspace by lowering the diaphragm and reducing visual interference by “smoke” from cauterization. This also facilitates dissection of hilar structures and the oblique fissure.
For lobectomies, the surgical procedure is systematized in order to minimize intraoperative lung manipulation. The first step is to section the pulmonary ligament. Next, in a posterior and superior direction, we perform a mediastinal and hilar lymphadenectomy whilst dissecting the elements of the hilum. This dissection is performed in a clockwise manner for right resections and counterclockwise for left resections, finalizing at the superior pulmonary vein. Then we open the fissure and complete the dissection of the remaining hilar structures. After that, the vessels and bronchus are stapled and the surgical specimen removed through the assistants port (Figure 2). To that end, the lower port is used as originally described by Dylewski et al. Given that the lower port is located at the transition between the diaphragm and the chest wall and below the 10th rib, the resected lobe can be removed without the limitation imposed by the ribs. Larger specimens can be removed this way as well, resulting in less pain.

From April/2015 to July/2018 we performed 187 pulmonary resections, being 142 lobectomies, 1 bilobectomy, 3 sleeve lobectomies and 41 sublobar resections (Table 1). Median operative time was 185 minutes (IQR 150–240). There was only one conversion to thoracotomy due to strong pleuropulmonary adhesions leading to continuous bleeding from the lung parenchyma that prevented the operation to progress adequately. Thirty-five patients coursed with postoperative complications but only 1 eventually died. The most frequent complication was prolonged air leak (16 patients). Median hospital stay was 3 days. Upon histology surgical margins were free in all but one patient and the median number of lymph nodes dissected was 11 (IQR 7–15).
Table 1
| Pulmonary resections (N=146) |
| Lobectomies (n=142) |
| RUL (n=55) |
| RML (n=5) |
| RLL (n=29) |
| LUL (n=24) |
| LLL (n=29) |
| Bilobectomy (1 RUL + RML) |
| Sleeve lobectomies (n=3) |
| RUL (n=2) |
| LUL (n=1) |
| Etiology |
| Indeterminate (n=19) |
| Benign (n=2) |
| Malignant (n=125) |
| Adenocarcinoma (n=95) |
| SCC (n=13) |
| Carcinoid (n=11) |
| Others (n=6) |
RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; SCC, squamous cell carcinoma.
Mediastinal tumor resections
For anterior mediastinal resections the patient is positioned in a 30-degree antidecubitus position with single lung ventilation. Laterality is defined after imaging evaluation but we favor a left-sided approach whenever possible. The first trocar is positioned in the fourth intercostal space at the anterior axillary line and is used for the camera and CO2 insufflation. The second trocar is placed in a cranial position in the third intercostal space at the anterior to middle axillary line. The third trocar is located caudally in the fifth intercostal space at the mid-clavicular line (Figure 3). For anterior tumors, the phrenic nerve is then identified and serves as reference throughout the dissection (Figure 4). As for posterior and middle mediastinum lesions we use a classic lateral decubitus positioning and trocar positioning similar to what we described for the pulmonary resections.

From November/2015 to July/2018 we performed 42 robotic mediastinal resections (Table 2). Median operative time was 120 minutes (QR 90–140). There were no intraoperative complications and only one postoperative mild complication (respiratory distress). Median hospital stay was 2 days.
Table 2
| Mediastinum (N=42) |
| Localization |
| Anterior (n=27) |
| Middle (n=5) |
| Posterior (n=10) |
| Etiology |
| Thymoma (n=12) |
| Cists (n=11) |
| Schawnnoma (n=2) |
| Other (n=17) |
Conclusions
MIS is well established in the field of thoracic surgery but despite its advantages, open thoracotomy remains the most common approach worldwide. In this scenario, RATS is becoming a very attractive form of MIS as it overcomes some of the problems imposed by VATS. Though many groups in North America and Europe have already described a mature experience with this method, the implementation of robotic programs in developing countries is still at its initial stages.
In Brazil, this method has not been thoroughly explored and there are only a few studies published on the subject. However, the experience with robotic surgery and the number of robotic platforms is growing steadily in our country and we have started performing robotic operations from the beginning of 2015 with very satisfactory results that we showed here.
Since then we have performed more than 200 operations, and our experience indicates that, with adequate training, results equivalent to VATS can be obtained with a short learning curve. We had a very low conversion rate and low morbidity from both lung and mediastinal resections. Furthermore, after overcoming the initial learning curve we started to decrease our operative time and accept more challenging cases such as sleeve lobectomies and bigger tumors while maintaining good outcomes. This meant we could offer MIS to a wider range of patients that otherwise would have had an open thoracotomy.
Even though this reflects the experience of a single group with RATS, we believe that these results can be reproduced, provided that adequate training of a multidisciplinary team can be achieved. We also think that this platform has great potential to grow in South America and especially in Brazil, since there is already an installed base of robots in the country and the expectancy to increase those numbers in the near future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Minimally Invasive Surgery - Robotics and VATS in Brazil”. The article has undergone external peer review.
Conflicts of Interest: The series “Minimally Invasive Surgery - Robotics and VATS in Brazil” was commissioned by the editorial office without any funding or sponsorship. RMT served as the unpaid Guest Editor of the series and reports educational grants from Johnson & Johnson, Medtronic and H. Strattner/Intuitive. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg 1993;56:1248-52; discussion 1252-3. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Hanna WC, de Valence M, Atenafu EG, et al. Is video-assisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy? Eur J Cardiothorac Surg 2013;43:1121-5. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-7.e1. [Crossref] [PubMed]
- Terra RM, Kazantzis T, Pinto-Filho DR, et al. Anatomic pulmonary resection by videoassisted thoracoscopy: the Brazilian experience (VATS Brazil study). J Bras Pneumol 2016;42:215-21. [Crossref] [PubMed]
- Tsukazan MTR, Terra RM, Vigo A, et al. Video-assisted thoracoscopic surgery yields better outcomes than thoracotomy for anatomical lung resection in Brazil: a propensity score-matching analysis using the Brazilian Society of Thoracic Surgery database. Eur J Cardiothorac Surg 2018;53:993-8. [Crossref] [PubMed]
- Ettinger S, Wood DE, Aisner DL, et al. Non-small cell lung cancer, Version 5.2017. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Serder CW, Salati M, Kozower BD, et al. Variation in Pulmonary Resection Practices Between The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons General Thoracic Surgery Databases. Ann Thorac Surg 2016;101:2077-84. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Non-small Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: Are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robotassisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Wei S, Chen M, Chen N, et al. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2017;15:98. [Crossref] [PubMed]
- Emmert A, Straube C, Buentzel J, et al. Robotic versus thoracoscopic lung resection: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7633 [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent premier data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of videoassisted thoracoscopic surgery and robotic approaches for clinical stage i and stage ii non-small cell lung cancer using the Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- 2017 Annual Report. Available online: www.intuitivesurgical.com.
- Martins Rua JF, Jatene FB, de Campos JR, et al. Robotic versus human camera holding in video-assisted thoracic sympathectomy: a single blind randomized trial of efficacy and safety. Interact Cardiovasc Thorac Surg 2009;8:195-9. [Crossref] [PubMed]
- Sardenberg RAS, Abadalla RZ, Abreu IRLB, et al. Robotic thymectomy for myasthenia gravis. Jornal Brasileiro de Pneumologia 2011;37:694-6. [Crossref] [PubMed]
- Santos RS, Cellulare AL, da Silva Costa A Jr, et al. Cardiothoracic robotic surgery in Brazil: lessons learned so far. 2013 ISMICS Annual Meeting. 12-15 June 2013, Hilton Prague, Prague, Czech Republic.
- Terra RM, Araujo PHXN, Lauricella LL, et al. Robotic pulmonary lobectomy for lung cancer treatment: program implementation and initial experience. J Bras Pneumol 2016;42:185-90. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg. 2011;23:36-42. [Crossref] [PubMed]
- Terra RM, Gouvêa FM, Araújo PH, et al. RATS left upper lobectomy. Asvide 2019;6:010. Available online: http://www.asvide.com/article/view/29601
- Terra RM, Gouvêa FM, Araújo PH, et al. Resection of mediastinal metastasis of thyroid carcinoma. Asvide 2019;6:011. Available online: http://www.asvide.com/article/view/29602
Cite this article as: Terra RM, Gouvêa FM, Araújo PHXN, Haddad R, Pêgo-Fernandes PM. Robotic-assisted thoracic surgery in Brazil, a review of the literature and our current experience. J Vis Surg 2019;5:15.