
Management of intraoperative bleeding in Korea
Introduction
Many reports have described various postoperative complications after lung cancer surgery. However, serious surgical errors may take place intraoperatively, and they can result in mortality or severe morbidity. In major lung resection, technically challenging events may occur during minimally invasive surgery (MIS) or thoracotomy. However, they are more technically difficult in MIS than in thoracotomy. According to a dedicated thoracic surgical center, catastrophic complications, including main pulmonary artery and main pulmonary vein transection requiring re-anastomosis, unplanned pneumonectomies, unplanned bilobectomy, tracheoesophageal fistula, membranous airway injury to the bronchus intermedius, complete staple line disruption of the inferior pulmonary vein, injury to the azygos/superior vena cava junction, and splenectomy, occurred in 1% of patients (1). In Korea, most general thoracic surgeries are performed minimally invasively (Table 1). Herein, we describe our expert experience in managing intraoperative bleeding during MIS.
Table 1
| Types | Multi-portal | Uni-portal | Robotic |
|---|---|---|---|
| The number of ports | 2–4 | 1 | 4–5 |
| Technical characteristics in intraoperative bleeding | (I) Multi-directional stapling or suturing; (II) easy to convert to thoracotomy |
(I) Mainly anterior approach; (II) converted to multi-portal VATS or thoracotomy |
(I) Multi-directional stapling or suturing; (II) stable compression; (III) various instrument utilization |
VATS, video-assisted thoracic surgery.
Pre-operative preparation
As with other complications, prevention is the most important step, and surgeons should be aware of various situations that can increase the risk of pulmonary vessel injury (1,2). Less experienced surgeons may apply excessive tension on the pulmonary vessels without realizing it. They are usually too focused on the target area to notice that they are stretching a pulmonary vessel that is out of surgical view. The lack of three-dimensional perception can also lead to erroneous instrumentation that results in serious hemorrhage.
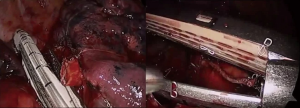
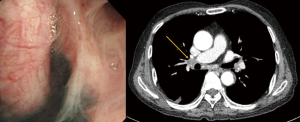
Pulmonary artery injury can also occur while encircling pulmonary arteries with an instrument without identifying the proper plane. Inexperienced movement of instruments through small ports, such as careless use of electrocautery or unstable placing and firing of the staples, can also cause bleeding. New robotic staples should be carefully applied as the movements of the anvil and jaw may be different from those of conventional endostaples. Surgeons should train themselves to learn how to avoid such errors (Figure 1). However, even experienced surgeons cannot completely prevent serious pulmonary artery injuries. Probably the most difficult situation in Korea is when calcified lymph nodes are trapped between the pulmonary artery and bronchus (Figure 2). Anthracotic and calcified lymph nodes often make dissection difficult as those lymph nodes are tightly adhered to the bronchus and pulmonary artery. Surgeons can usually predict such lymph nodes beforehand by carefully reviewing computed tomography (CT) scans and positron emission tomography-computed tomography (PET/CT) images. Preoperative bronchoscopy occasionally shows multiple anthracotic pigmentation of the area where the lymph node calcification was observed on the imaging studies (Figure 3). Based on Korean patient data, 14.3% showed bronchial anthracotic pigmentation on bronchoscopy, and 30.8% showed microscopic anthracotic pigmentation on endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) specimen (4). Typical CT findings of anthracotic lymph nodes include enlarged lymph nodes with high attenuation (calcification) and adjacent bronchial wall thickening (Figure 3) (5-7). The anthracotic lymph nodes are metabolically active with high fluorodeoxyglucose (FDG) uptake; however, they are histologically benign in general. In addition, a lack of understanding the variations of intrathoracic anatomy can also cause serious complications. Such structural variations should be acknowledged preoperatively by carefully reviewing the CT scans. Bleeding can also occur from the pulmonary parenchyma, especially after performing wedge resection in patients whose pulmonary arterial pressure is increased. For such patients, the staple lines should be oversewn meticulously, as the elevated pulmonary arterial pressure may cause postoperative bleeding.

Tips, tricks and pitfalls
Despite all the efforts made to avoid pulmonary vessel injuries, disasters can happen, and pulmonary vessel injuries can be life-threatening. In cases of major pulmonary arterial bleeding, we recommend following four crucial steps.
First, the bleeding focus should be compressed with either a sponge stick or alternative instruments, in a gentle but secure manner. Attention should be paid to avoid applying too much pressure, as this may enlarge the tear site. When carbon dioxide insufflation is used intraoperatively, the suction of blood must be conducted carefully because the negative pressure of suction will cause the thoracic cavity to collapse. Occasionally, bleeding control with compression can be achieved by simply flipping the lung to the other side. If bleeding stops, one can continue working on the other area, and over time, the bleeding can be controlled naturally.
Second, surgeons should remain calm and inform all personnel in the operating room of the situation so that they can be prepared to provide additional treatment. For example, it is necessary to inform anesthesiologists to prepare for massive transfusion, to request nurses to prepare for conversion to open thoracotomy, and to provide endoscopic instruments for suturing and vascular clamping. In particular, close cooperation between the surgeon operating the robotic console and the bedside assistant is essential for robotic surgery.
Third, surgeons must decide whether to convert to open thoracotomy or to continue performing MIS. Usually, the easiest way to avoid lethal bleeding is to convert from MIS to thoracotomy. It is important to decide when to covert. During conversion to open thoracotomy or sternotomy, the assistant and the camera should concentrate on maintaining proper compression of the bleeding focus. If surgeons have experience with performing at least 50 cases of thoracoscopic lobectomies, they might be justified in trying to control bleeding during MIS (8-10). Conversely, surgeons without sufficient experience with MIS should not continue to perform MIS because attempts to repair a pulmonary artery injury can sometimes make the situation worse.
Fourth, if one decides to repair the site of bleeding in MIS, various kinds of hemostatic material, such as a fibrin sealant with polyglycolic acid felt (Neoveil®, Gunze, Tokyo, Japan), which compresses the edge of the raw surface and acts as a sealant absorbable material, or fibrinogen-based collagen fleece (TachoSil®, Takeda Austria GmbH, Linz, Austria), are very useful (11). The application of topical hemostatic material on the injured point may help decrease the rate of bleeding, and can sometimes stop the bleeding (Figures 2,4). Although hemostatic materials are easy to use, they can get become detached by suction or excessive retraction of the lung. To avoid this, it is recommended to apply a second layer of coverage using a larger material. If endoscopic suturing is required, it is sometimes necessary to clamp the proximal pulmonary artery to avoid massive bleeding and to obtain better visualization of the tear site.

When the decision is made to proceed with MIS and not to convert to thoracotomy, several tips should be kept in mind. First, when the calcified lymph nodes seem benign either in the clinical impression or in results from a frozen biopsy report, one can decide to leave the lymph node and dissect the distal points of the vascular branch. Even if bleeding occurs, the amount of bleeding can be minimized and easily controlled. Second, one can break the calcified lymph node itself by using either sharp scissors or electrocautery. As one keeps dissecting, the lymph node will gradually become separated, exposing the space between the pulmonary artery and bronchus. However, this is usually time-consuming, and there is a significant risk of pulmonary arterial rupture due to the adventitia being usually thin. To avoid catastrophic hemorrhage, it is safer to encircle the main pulmonary artery before initiating dissection.
Following the general rules of surgery, such as having a sufficient dissection plane, maintaining clear visualization of the target structure, paying attention to the tip of sharp devices, and setting all necessary surgical instruments on the table, are important to prevent intraoperative bleeding. If surgeons are less experienced, it is always a good idea to ask experienced surgeons for help. In addition, providing frequent education for surgical team members is important to prevent catastrophic bleeding.
Procedure
Bleeding control during multiportal MIS
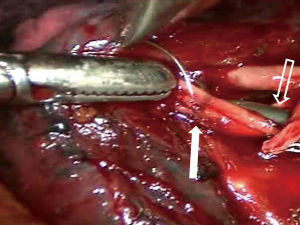
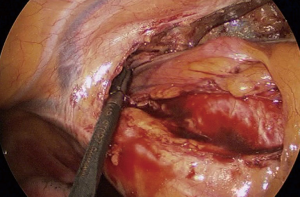
Although several methods of MIS have been used, including multiportal or uniportal video-assisted thoracic surgery (VATS) and robotic surgery in Korea, multiportal VATS has been the most popular approach. Various techniques have been introduced to manage intraoperative bleeding during VATS (13-15). In most cases, surgical clips, electrocautery, or diverse energy modalities, such as ultrasound shear and the bipolar sealing device, are effective for managing minor bleeding from the stapling line, small distal vascular injury, and parenchymal laceration. Small-sized vascular injury can be simply sutured or managed with hemostatic materials without proximal control of the vessel. When major bleeding occurs, it is advantageous to perform multiportal VATS because one can immediately and properly compress the bleeding focus from multiple directions without causing collision of the devices. Various instruments can then be introduced through the remaining ports to control the bleeding. For example, while a suction device compresses the bleeding point of the pulmonary artery from the posterior port, needle holder and forceps can be entered through the anterior working windows to suture the tear site. Sometimes, vascular clamps or small bulldog clamps can be used to ensure a clear view of the rest of the tear site while suturing the pulmonary artery (Figure 5). In a situation where carbon dioxide gas insufflation is used, suction devices and large sponge sticks may not be suitable. For example, when innominate vein laceration occurs during VATS thymectomy, clearing the blood with a suction device will compromise the working space and subsequently make the situation worse. In this situation, we recommend using adjacent thymic tissue or lung parenchyma to compress the bleeding point (Figure 6). After obtaining instant compression of the injured area, one can place an additional port or convert to sternotomy safely.
Bleeding control during uniportal MIS
Recent advancement of surgical instruments and increased experience with VATS rapidly enabled uniportal VATS to be used in complex procedures (16,17). Although the surgical view in uniportal MIS is similar to that during thoracotomy, understanding the limitations of instruments and preventing collisions between them require a longer learning period, as the singular surgical window contributes to a poor ergonomic condition (18,19). Consequently, managing intraoperative bleeding of major vessels is sometimes more challenging during uniportal VATS than during multiportal VATS. If significant bleeding occurs, the bleeding point must be compressed first with a sponge stick, and then the surgeon should make the decision whether to continue uniportal VATS or to convert to either multiportal VATS or open thoracotomy.
When the decision is made to convert to multiportal VATS, one or two additional ports must be placed at the seventh or eighth intercostal space. Some surgeons prefer making an additional port along the same intercostal space to minimize the patient’s postoperative pain. Subsequently, either one of the endoscopic instruments or the camera is moved to the second port depending on the situation. This provides additional freedom in the instruments’ movement and thus, facilitates bleeding control. If the decision is made to perform open thoracotomy, the uniportal incision is extended along the same intercostal space. If the decision is made to not convert to multiportal VATS, one can use Hemoclips, Hem-o-lok clips, or direct sutures. In Korea, TachoSil® is commercially available. One of the advantages of using TachoSil® during uniportal VATS is that it is easy to introduce it and apply it through one small incision. Additionally, it has an advantage over Hemoclips or Hem-o-lok clips, as the vessels controlled by TachoSil® can be safely divided by using endostaplers. Hemostatic gauze can also be used to control small branch injuries. The QuikClot combat plus (Z-MEDICA, LLC, Wallingford, CT, USA) is one of the most popular types of hemostatic gauze that contains kaolin, an adsorbent that promotes coagulation by creating a hemoconcentration effect, thus facilitating the aggregation of platelets and clotting factors (Figure 4) (20).
Bleeding control during robotic surgery
There are several differences between robotic surgery and conventional VATS. The lack of tactile sensation requires surgeons to perform dissection and encircling of the vessel carefully based on the visual sense alone. When the surgeon is sitting on the robotic console away from the operating field, it is sometimes difficult to control the bleeding promptly when significant bleeding events occur. Therefore, all the surgical team members have to be prepared and fully understand their role in order to be actively involved to achieve successful bleeding control (21). Minor bleeding such as diffuse oozing or bleeding from the small pulmonary arterial branches can be easily controlled by various methods, including compression with thrombostatic materials (22), clipping, or suturing (23). Although robotic suction devices are available, performing suction at bedside is more effective in a situation of active bleeding (Figure 7). Some experienced robotic surgeons may clamp the proximal portion of the bleeding vessel with Cartier forceps and use the robot arm as if it were a vascular clamp. The bleeding site may be sutured successfully using the second or third arm.

If bleeding control via robotic MIS fails, the surgeon should develop a clear strategy on how to convert to thoracotomy. Thoracotomy should be considered as a backup plan during robotic surgery, especially when the risk of bleeding is high. Some surgeons in Korea prefer to make the surgical ports and working window along the fourth or fifth intercostal space to ensure prompt conversion to thoracotomy. When the decision is made to convert to open thoracotomy, it is preferred to have the bedside assistant compress the bleeding point and remove all docked robotic arms, except for the camera, from the patient. If one of the robot arms is used to control the bleeding, it is better to have another experienced surgeon perform thoracotomy. During conversion, the robotic scope should be used to observe the bleeding points.
Conclusions
When major bleeding occurs, a surgeon should manage it in a suitable way. Furthermore, providing training on surgical techniques of MIS and education of anatomical variations, in addition to enforcing stringent observances of the surgical principle of bleeding control, are mandatory to overcome intraoperative complications. The most important aim of any operation is patient safety, and ensuring patient safety may require conversion to open thoracotomy to prevent intraoperative bleeding.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Seshiru Nakazawa and Kimihiro Shimizu) for the series “Emergency Response to Intraoperative Bleeding” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.12.03). The series “Emergency Response to Intraoperative Bleeding” was commissioned by the editorial office without any funding or sponsorship. Hyun Koo Kim serves as an unpaid editorial board member of Journal of Visualized Surgery from Sep 2018 to Aug 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Individual informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg 2011;142:1412-7. [Crossref] [PubMed]
- Yano M, Takao M, Fujinaga T, et al. Adverse events of pulmonary vascular stapling in thoracic surgery. Interact Cardiovasc Thorac Surg 2013;17:280-4. [Crossref] [PubMed]
- Park S, Kim HK, Park SY, et al. Anthracotic lymph nodes often make dissection difficult, as those lymph nodes are tightly adhered to the bronchus and pulmonary artery. The bleeding from the pulmonary artery was controlled with TachoSil®. VATS left upper lobectomy was completed without open conversion. Asvide 2018;5:914. Available online: http://www.asvide.com/article/view/28942
- Park YS, Lee J, Pang JC, et al. Clinical implication of microscopic anthracotic pigment in mediastinal staging of non-small cell lung cancer by endobronchial ultrasound-guided transbronchial needle aspiration. J Korean Med Sci 2013;28:550-4. [Crossref] [PubMed]
- Kim HY, Im JG, Goo JM, et al. Bronchial anthracofibrosis (inflammatory bronchial stenosis with anthracotic pigmentation): CT findings. AJR Am J Roentgenol 2000;174:523-7. [Crossref] [PubMed]
- Dhar A, Sikund K, Lall A, Aggarwal B. Radiological spectrum of anthracofibrosis: A series of 40 patients with computed tomography, bronchoscopy, and biopsy. Indian J Radiol Imaging 2017;27:397-403. [Crossref] [PubMed]
- Mirsadraee M. Anthracosis of the lungs: etiology, clinical manifestations and diagnosis: a review. Tanaffos 2014;13:1-13. [PubMed]
- Puri V, Gaissert HA, Wormuth DW, et al. Defining Proficiency for Society of Thoracic Surgeons Participants Performing Thoracoscopic Lobectomy. Ann Thorac Surg 2018; [Epub ahead of print]. [PubMed]
- McKenna RJ Jr. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- Ferguson J, Walker W. Developing a VATS lobectomy programme--can VATS lobectomy be taught? Eur J Cardiothorac Surg 2006;29:806-9. [Crossref] [PubMed]
- Hickman DA, Pawlowski CL, Sekhon UD, et al. Biomaterials and Advanced Technologies for Hemostatic Management of Bleeding. Adv Mater 2018;30. [PubMed]
- Park S, Kim HK, Park SY, et al. Hemostatic gauze (QuikClot combat plus) can also be used to control small branch injuries. Asvide 2018;5:915. Available online: http://www.asvide.com/article/view/28943
- Yamashita S, Tokuishi K, Moroga T, et al. Totally thoracoscopic surgery and troubleshooting for bleeding in non-small cell lung cancer. Ann Thorac Surg 2013;95:994-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Stupnik T, Fernandez R, de la Torre M, Velasco C, Yang Y, et al. Intraoperative bleeding control by uniportal video-assisted thoracoscopic surgerydagger. Eur J Cardiothorac Surg 2016;49:i17-24. [PubMed]
- Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. [Crossref] [PubMed]
- Wu CF, Fernandez R, Mercedes T, et al. Mid-term survival outcome of single-port video-assisted thoracoscopic anatomical lung resection: a two-centre experience. Eur J Cardiothorac Surg 2018;54:252-9. [Crossref] [PubMed]
- Han KN, Kim HK, Choi YH. Midterm outcomes of single port thoracoscopic surgery for major pulmonary resection. PLoS One 2017;12:e0186857. [Crossref] [PubMed]
- Su WL, Huang JW, Wang SN, et al. Comparison study of clinical outcomes between single-site robotic cholecystectomy and single incision laparoscopic cholecystectomy. Asian J Surg 2017;40:424-8. [Crossref] [PubMed]
- Grochola LF, Soll C, Zehnder A, et al. Robot-assisted single-site compared with laparoscopic single-incision cholecystectomy for benign gallbladder disease: protocol for a randomized controlled trial. BMC Surg 2017;17:13. [Crossref] [PubMed]
- Granville-Chapman J, Jacobs N, Midwinter MJ. Pre-hospital haemostatic dressings: a systematic review. Injury 2011;42:447-59. [Crossref] [PubMed]
- Louie BE. Catastrophes and complicated intraoperative events during robotic lung resection. J Vis Surg 2017;3:52. [Crossref] [PubMed]
- Kocher GJ, Schmid RA, Melfi FM. Robotic lobectomy: tips, pitfalls and troubleshooting. Eur J Cardiothorac Surg 2014;46:e136-8. [Crossref] [PubMed]
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Park S, Kim HK, Park SY, et al. Bleeding from the azygos vein is shown during robotic esophagectomy. Suction performed by the bedside surgeon causes the thoracic cavity to collapse; therefore, compression with gauze is applied repeatedly. The bleeding focus is ligated with a Hem-o-lok clip. Asvide 2018;5:916. Available online: http://www.asvide.com/article/view/28985
Cite this article as: Park S, Kim HK, Park SY, Kim HK, Kim YT. Management of intraoperative bleeding in Korea. J Vis Surg 2018;4:252.


