Response to intraoperative bleeding during video-assisted thoracoscopic surgery
Introduction
In recent years, thoracoscopic surgery has become increasingly widely used because of improvements in optical and surgical instruments and advances in surgical technical skills. Although minimally-invasive surgeries including video-assisted thoracoscopic surgery (VATS) and robot-assisted thoracoscopic surgery have gained popularity in the management of primary lung cancer and other chest diseases, they are of little worth unless adequately and safely performed. We thoracic surgeons should remember that these procedures have the possible risk of becoming catastrophic and “maximally-invasive” surgery. Avoiding bleeding troubles in thoracoscopic surgery is important; however, knowing how to manage the bleeding might be more important.
In this review article, we discuss how to respond to and manage unexpected intraoperative bleeding in thoracoscopic surgery and outline the essentials to safely perform thoracoscopic surgery.
Review
Characteristics of pulmonary vessel injury
Causes of pulmonary vessel injury can be classified into those (I) at dissection and (II) at cutting. Representative vessel injury at dissection is often caused by infiltrating lymph nodes due to silicotic, calcific, or metastatic lymph nodes, or by forcible tissue trafficking. Representative vessel injury at cutting is due to technical mistakes during ligation, or the use of staplers or energy devices. According to the National Institutes of Health’s Common Terminology Criteria of Adverse Events version 5.0 (1), Grade 4 is life-threatening bleeding, and Grade 5 is deadly bleeding (Table 1). If so, how much is bleeding over Grade 4? If a person loses approximately one third of their circulating blood volume, he or she will go into hemorrhagic shock. A male with a body weight of 60 kg has a circulating blood volume of 4,800 mL (approximately 8% of the body weight); therefore, losing approximately 1,600 mL (1/3 of 4,800 mL) is sufficient to cause hemorrhagic shock. Because blood flow in the pulmonary artery is the same as cardiac output (3.6 to 5.8 L/min =3,600 to 5,800 mL/60 seconds), blood loss reaches 1,600 mL in about only 20 seconds. Considering this, it is apparent that it may be easy to cause instant catastrophic bleeding if we damage major trunks or thick branches of the pulmonary artery, especially by misfiring staplers or causing pull-out injury in the pulmonary artery branch. Generally, it is probable that bleeding exceeding 1,500 mL is Grade 4 or greater, and bleeding above 1,000 mL is a dangerous situation.
Table 1
| CTCAE term | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Definition |
|---|---|---|---|---|---|---|
| Intraoperative arterial injury | Primary repair of injured organ/structure indicated | Partial resection of injured organ/structure indicated | Complete resection or reconstruction of injured organ/structure indicated; limiting self-care ADL | Life-threatening consequences; urgent intervention indicated | Death | A finding of damage to an artery during a surgical procedure |
| Intraoperative hemorrhage | – | – | Postoperative invasive intervention indicated; hospitalization | Life-threatening consequences; urgent intervention indicated | Death | A finding of uncontrolled bleeding during a surgical procedure |
ADL, activities of daily living.
In thoracoscopic surgeries, more than two third of vessel injuries involve branches of the pulmonary arteries and veins in thoracoscopic surgeries (2). These vessels are usually thin and susceptible to pull-out injury, and pulmonary arteries develop atherosclerosis with advancing age because collagenous fibers increase in internal media whereas elastic fibers and smooth muscle fibers decrease. However, we can easily control bleeding from pulmonary arteries because of its low pressure, and it is possible to resupply lost blood through the transfusion.
Responding to bleeding during VATS: controlling bleeding
The first thing to do before controlling bleeding is to calm yourself. We should never be upset and lose our composure. Absolutely, the most important thing during intraoperative bleeding is surgeons’ ability to evaluate a situation accurately.
The next step is to compress the bleeding site gently and softly for several minutes. We should use endoscopic sticks with soft cotton, gauze, or absorbent sponge to compress the bleeding site. We should avoid using sharpened instruments such as forceps or compressing the bleeding site firmly, even with soft cotton. It is also effective to rotate the lung parenchyma to the normal position and compress the bleeding site over it (3). If we cannot control the bleeding effectively because of its seriousness, instrumental inaccessibility, or poor visibility due to pleural adhesion and so on, we should never hesitate to convert to open thoracotomy.
Additionally, surgeons must inform anesthesiologists and operating room nurses about the bleeding, especially if severe hemorrhage occurs so that systemic blood volume can be maintained and the instruments for emergency thoracotomy can be promptly prepared. Because a clear field of view through the thoracoscope is easily obscured by blood spatter, it is necessary to clean thoracoscopic tips frequently and prepare two independent suction tubes. Moreover, it is also important to record scenes of hemorrhage for review using the thoracoscope.
Responding to bleeding during VATS: hemostasis
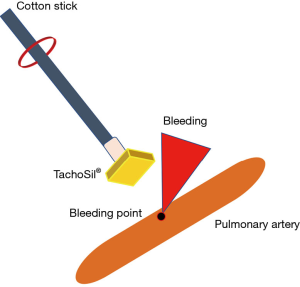
Many small bleeds can be stopped by compression alone; however, we should acquire proficiency in several hemostatic methods because we sometimes experience cases in which we struggle to achieve complete hemostasis. For temporal or complete hemostasis during thoracoscopic surgeries, we recommend applying a collagen patch coated with human fibrinogen and thrombin, TachoSil® (CSL Behring, King of Prussia, PA, USA), to the bleeding site. This can be cut into 1- or 2-cm squares and affixed with a drop of xylocaine jelly or saline solution on the surface before patching. We can carry this to the bleeding point using endoscopic cotton sticks and apply a patch to the point with compression for at least 3 minutes (Figure 1). This is an effective and useful way to achieve hemostasis in thoracoscopic surgeries, and complete hemostasis can be achieved without the need for any sutures in most bleeding cases (Figure 2). Regarding repairing vessel injuries, Ikeda et al. reported that elastic fibers and smooth muscle had completely regenerated in the medial and adventitial layer of the pulmonary artery in female beagles by 8 weeks after TachoSil® patch (5). If we feel it is hard to achieve complete hemostasis even using TachoSil®, we should immediately convert to open thoracotomy. Also, in the situation of bleeding from the root of first branches of pulmonary arteries, we should never hesitate emergency conversion and immediately try to clamp proximal side of the main pulmonary arteries. It would be more dangerous to continue to use the thoracoscopic approach during bleeding and continue with a hemostatic procedure in an unfamiliar situation.

Responding to bleeding during VATS: repairing and cutting injured vessels
To completely repair injured vessels, we must expose and mobilize the distal and proximal sides of the bleeding sites. Then we encircle and cross-clamp those vessels and continuously or interruptedly stitch up the injured sites with 5-0 or 6-0 non-absorbable monofilament suture. It is advisable to perform patch closure using autologous pericardium and so on if we find the relatively large injured area. When cutting the injured vessels, we do not need to remove the TachoSil® if we have obtained successful hemostasis with it. We can even suture or cut the injured vessels over it using endostaplers under safe conditions. As a matter of course, we should repair or cut injured vessels while controlling bleeding, and it is never safe to do so in the bloody operative field (Figure 3).

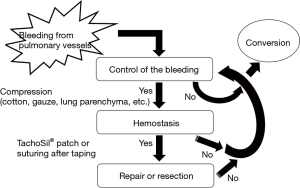
Figure 4 shows our algorithm for unexpected intraoperative bleeding during VATS. As shown in the figure, it is important to envision the cycle to control intraoperative bleeding and predict the timing of conversion to open thoracotomy (Figure 4).
Responding to bleeding: indication and timing of conversion to open thoracotomy
There is an obvious difference in the indication and timing of conversion to thoracotomy among institutions and individual surgeons. Hanna et al. analyzed reasons for conversion and classified them into four types: (I) intraoperative complications (such as bleeding); (II) technical problems (such as calcified lymph nodes); (III) anatomical problems (such as unclear vision), and (IV) oncological conditions (such as metastatic lymph nodes or tumor invasion) (7). Gazala et al. also demonstrated the importance of conversion because of intraoperative bleeding in thoracoscopic surgeries (8). Samson et al. stated that the presence of calcified lymph nodes was a significant predicting factor for conversion to open thoracotomy in VATS (9). Yamashita et al. also analyzed the injury portions, recovery approaches, and hemostatic procedures of patients who underwent thoracoscopic surgery and had intraoperative vessel injuries (10). They found that injury in pulmonary artery branches was the most frequent cause of conversion to thoracotomy, and that bleeding could be adequately controlled under minithoracotomy.
Although not only indication and timing of conversion but also conversion methods differ among institutions, it is important to perform it as quickly as possible, accurately localize the bleeding site, and promote recovery with the minimal amount of bleeding.
Conclusions
We briefly outlined how to respond to intraoperative bleeding to perform safe thoracoscopic surgery. It is important to master the basic surgical procedures and to carry out a simulation to train for adverse events in VATS. We also should keep in mind the algorithms for managing intraoperative bleeding during VATS.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Seshiru Nakazawa and Kimihiro Shimizu) for the series “Emergency Response to Intraoperative Bleeding” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.10.20). The series “Emergency Response to Intraoperative Bleeding” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schoen MW, Basch E, Hudson LL, et al. Software for Administering the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events: Usability Study. JMIR Hum Factors 2018;5:e10070 [Crossref] [PubMed]
- Japan Society for Endoscopic Surgery. 12th Nationwide Survey of Endoscopic Surgery in Japan J Jpn Soc Endosc Surg 2014;19:569-82. (in Japanese).
- Demmy TL, James TA, Swanson SJ, et al. Troubleshooting video-assisted thoracic surgery lobectomy. Ann Thorac Surg 2005;79:1744-52; discussion 1753.
- Haruki T, Nakamura H. Pull-out injury of a small branch of pulmonary artery of right upper lobe. Asvide 2018;5:845. Available online: http://www.asvide.com/article/view/28188
- Ikeda T, Miyata Y, Tsutani Y, et al. Fibrinogen/thrombin-based collagen fleece (TachoComb(R)) promotes regeneration in pulmonary arterial injury. Eur J Cardiothorac Surg 2012;41:926-32. [Crossref] [PubMed]
- Haruki T, Nakamura H. Injury of the right lower pulmonary vein by the electrocautery scalpel. Asvide 2018;5:846. Available online: http://www.asvide.com/article/view/28189
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5:S182-9. [PubMed]
- Gazala S, Hunt I, Valji A, et al. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:962-4. [Crossref] [PubMed]
- Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Moroga T, et al. Totally thoracoscopic surgery and troubleshooting for bleeding in non-small cell lung cancer. Ann Thorac Surg 2013;95:994-9. [Crossref] [PubMed]
Cite this article as: Haruki T, Nakamura H. Response to intraoperative bleeding during video-assisted thoracoscopic surgery. J Vis Surg 2018;4:232.