
Tips and tricks for entering a difficult chest via VATS
Introduction
Video-assisted thoracoscopic surgery (VATS) is widely accepted as an alternative approach for treating various types of thoracic disease. In our experience with VATS, severe adhesions between the lung and parietal pleura following infection or as a result of prior thoracic surgery can be problematic. On the other hand, intrathoracic adhesions can be peeled apart relatively simply via dissection under visual monitoring. In this article, we intend to introduce the tips and tricks derived from our experience for entering a difficult chest during VATS.
General VATS procedure tips
In our VATS procedures, we usually use a “confronting upside-down” monitor configuration (1). In brief, two monitors are installed on the cranial side of a patient, one of which is placed upside-down. Regardless of the side of the thorax being operated upon, a primary surgeon stands on the right side of a patient and both camera and second assistants on the left side. Each individual obtains a correct view of the surgical field in the confronting monitor (e.g., on the video monitor watched by surgeon, the left side of the image is the cranial side of a patient).
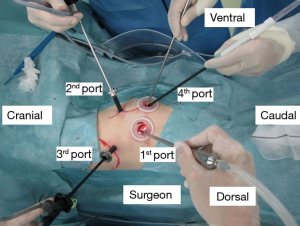
We usually use three ports (7-, 7-, and 15-mm sizes) for VATS wedge resection or mediastinal tumor resection and four ports (7-, 7-, 15-, and 30-mm sizes) for VATS segmentectomy or lobectomy in patients with malignant lung tumors to conduct both radical and safe procedures (1,2). A given operation is completely performed under thoracoscopic visualization; notably, all structures in the chest cavity are clearly visible using a high-definition camera with 30-degree lens (Figure 1).
Port creation in normal cases
The first step of port setting is port installation. Each port is installed at the center of the intercostal space to prevent postoperative neuralgia. After making a skin incision, the muscles are divided by electrocautery. Then, the parietal pleura is opened using a straight instrument without visualization, followed by insertion of the first port and determination of adhesion between the lung and parietal pleura. Other ports are installed under video monitoring (Figure 2). If adhesions in the thoracic cavity are detected, we dissect them via electrocautery and/or other energy-based instruments. If a blood vessel is included in the adhesion, we usually use a clip prior to dissection to prevent or mitigate postoperative bleeding following adhesiolysis. In addition, damage to local major vessels, such as the aorta, superior vena cava, or hemiazygos vein, should be carefully avoided. To avoid major vessel injury, we confirm the location of the sympathetic and phrenic nerves during surgery because these nerves are close to the major vessels. If we confirm them during adhesiolysis, we should pay attention to the major vessel. Essentially, a thorough understanding of the relevant anatomical structures is essential prior to performing adhesiolysis.

Suggestions regarding the approach for entering a difficult chest
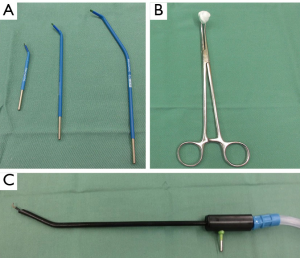
While opening the parietal pleura, if the sound of a patient’s inhalation is inaudible, this is noted as a sign of possible adhesion between the lung and parietal pleura. After setting the first port, we confirm the presence/absence of dense adhesions. If dense adhesions are identified around the first port, we peel them off in the direction of the second (camera) port using the tip of the scope. While entering a difficult chest, the first priority is to establish the second port. After setting the camera port, we can use electrocautery (20 to 40 W power) and swabs to peel off the adhesion near these ports. Electrocautery is advantageous in that the tip can be of varying lengths and can be bent as needed for use in a narrow space (Figure 3). However, when only two ports are installed, suction is difficult to use for removing smoke because the deflated lung tends to be re-inflated by the suction. Thus, the second priority is to place the third port. Until then, we attempt to limit the use of open electrocautery devices and suctionless endoscopic devices, despite the mild impairment in visualization by smoke (Figure 4). Following the creation of the third port, endoscopic electrocautery can be used with suction, allowing dense adhesions to be dissected under clear visualization. Owing to the possibility of lung injuries in these cases, we are equipped to repair such injuries with both suture and a polyglycolic acid sheet cover and with fibrin glue. In cases comorbid with inflammatory diseases such as tuberculosis and aspergillosis or re-operation, adhesion may be severe. In such situations, we attempt to peel the adhesion off in an extrapleural layer (Figure 5).


VATS-based adhesiolysis has some advantages. First, it can be performed under video monitor visualization, reducing blood loss and incidence and extent of lung injury relative to blind adhesiolysis. Second, endoscopic instruments can be used anywhere in the chest cavity; furthermore, such flexibility improves the selection of appropriate instruments, decreasing operative morbidities including postoperative bleeding and air leakage.
Conclusions
VATS is a useful approach, particularly in cases wherein an adhesion between the lung and parietal pleura has to be peeled off. While entering a difficult chest, we use open instruments (e.g., electrocautery) and swabs to effectively perform blunt dissection near the port. Adhesiolysis performed under video monitor visualization can prevent or mitigate injury to each structure in the thoracic cavity. Finally, in cases of damage, the injured lung should be adequately repaired, and in all cases, bleeding should be controlled to the best of one’s ability.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kamran Ali) for the series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.10.15). The series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mun M, Ichinose J, Matsuura Y, et al. Video-assisted thoracoscopic surgery lobectomy via confronting upside-down monitor setting. J Vis Surg 2017;3:129. [Crossref] [PubMed]
- Mun M, Nakao M, Matsuura Y, et al. Thoracoscopic segmentectomy for small-sized peripheral lung cancer. J Thorac Dis 2018;10:3738-44. [Crossref] [PubMed]
- Mun M, Ichinose J, Matsuura Y, et al. Port creation in a normal case (operation on the right side). Asvide 2018;5:833. Available online: http://www.asvide.com/article/view/27993
- Mun M, Ichinose J, Matsuura Y, et al. Port creation and adhesiolysis in a patient with dense adhesion. Asvide 2018;5:834. Available online: http://www.asvide.com/article/view/27994
- Mun M, Ichinose J, Matsuura Y, et al. Extrapleural dissection. Asvide 2018;5:835. Available online: http://www.asvide.com/article/view/27995
Cite this article as: Mun M, Ichinose J, Matsuura Y, Nakao M, Okumura S. Tips and tricks for entering a difficult chest via VATS. J Vis Surg 2018;4:227.


