Advanced hepatocellular carcinoma with portal tumor thrombosis in cirrhotic patient: laparoscopic right hepatectomy with vascular reconstruction
Introduction
Hepatocellular carcinoma (HCC) is the most frequent primary hepatic malignancy and is frequently associated with cirrhosis (1). Tumor vascular invasion may be present in many cases of HCC. Although it is associated with poor prognosis, liver resection (LR) may result in improved long-term survival when compared to other therapies (2).
Performing a hepatectomy in a HCC patient with cirrhosis is a challenging task due to the risk of postoperative liver failure and increased morbidity and mortality rates (3). Laparoscopic liver resections (LLR) emerged as an alternative as it allows lower postoperative liver failure and morbidity rates, and has been widely adopted (3-5).
However, there are very few reported cases of LLR in cases of portal vein tumor thrombosis because of the technical challenges and risk of postoperative complications involved (6).
Case presentation
Patient and pre-operative work-up
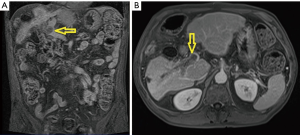
A 72-year-old male with alcoholic cirrhosis presented with upper abdominal pain in the last 2 weeks. A magnetic resonance image (MRI) study disclosed a 10 cm lesion suggestive of HCC on the right hepatic lobe with tumor thrombosis of the right portal vein (Figure 1). Total hepatic volume was 1,083 mL and the left liver (the future remnant liver) presented 433.8 mL. As symptoms lasted for only 2 weeks, we assumed that the right portal vein thrombosis was recent (left liver hypertrophy could happen), and taking under consideration the high mortality rates of a right hepatectomy on a cirrhotic patient, initial treatment was chemotherapy with sorafenib.
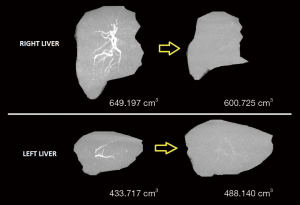
One month after sorafenib was started a computed tomography (CT-scan) was performed. HCC dimensions persisted but areas of necrosis appeared. Due to right portal vein thrombosis, left liver increased from 433.8 to 488 mL (13%) and the right liver (resected on a right hepatectomy) reduced from 649.2 to 600.8 mL (7.5%) (Figure 2).
As the liver showed signs of regenerative capacity, a laparoscopic right hepatectomy with portal vein thrombectomy and reconstruction was proposed.
Pre-operative preparation
As pre-operative preparation an electrocardiography, an echocardiogram and laboratory tests including liver function tests were performed.
Equipment preference card
The endoscopic materials used for the procedure were: a 30-degree, 10-mm endoscope (Storz. Tuttlingen, Germany), the SDC Ultra HD Information Image Management System Endoscopy (Stryker. Kalamazoo, USA), 1288 HD High definition Camera (Stryker, Kalamazoo, USA), L9000 Led Light Source (Stryker. Kalamazoo, USA), and Penumo Sure High Flow Insuflator (Stryker, Kalamazoo, USA). The harmonic scalpel used was the Ultracision Harmonic Ace® (Ethicon. Somerville, USA) and we used the Echelon Flex™ Endopath® Stapler (Ethicon. Somerville, USA).
Procedure
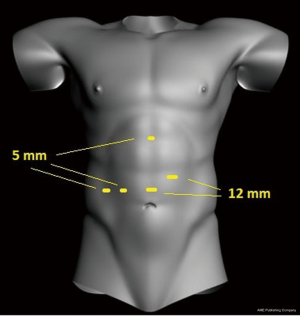
Two 12 mm and three 5 mm trocars were used (Figure 3). At inspection there were signs of portal hypertension and cirrhosis and the right liver was atrophied (Figure 4A). The right hepatic artery and bile duct were transected. Then, the main portal vein was dissected close to its bifurcation and encircled. After some parenchymal transection, we proceeded to the treatment of the portal vein tumor thrombosis. Instead of performing a left hemi-Pringle, we performed an isolated clamping of the left portal vein leaving the left hepatic artery open to lessen remnant liver warm ischemia. Then, the main portal vein was clamped (Figure 4B). The right portal vein and a lateral portion of the main portal vein were transected.
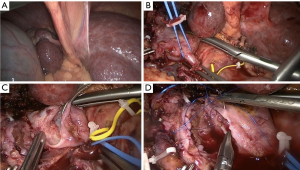
Tumor thrombectomy of the main portal vein was performed (Figure 4C). A running suture was run up the defect created (Figure 4D). Clamps were removed. Then, parenchymal transection was performed in the plane correspondent to a right hepatectomy. The right hepatic vein was transected with an endoscopic stapler. Specimen was retracted through a suprapubic incision. A Blake drain was put in place and the procedure completed.
Operative time was 500 minutes. Estimated blood loss was 275 milliliters (Figure 5).

Role of team members
The team consisted of four gastrointestinal surgeons with expertise in hepato-bilio-pancreatic surgery and an anesthesiologist. One surgeon performed all the steps of the procedure. Another surgeon was the camera assistant during the entire procedure and the other two surgeons were assistants.
Post-operative management
Patient developed mild ascites, managed with diuretics for one month. He presented no other signs of liver dysfunction. He stayed on the intensive care unit for two days and was discharged from hospital on postoperative day 10. A CT scan was performed one week after the surgery and disclosed normal portal vein flow. Pathology came out as high degree HCC with vascular invasion. Margins were free from tumor. One year after a CT-scan showed no signs of tumor recurrence.
Discussion
HCC is the fifth most common malignancy in the world and ranks third in cancer-related deaths (8). During the last few decades multiple therapeutic modalities for the treatment of HCC have emerged, such as locoregional therapies (ablation, embolization, chemoembolization, and percutaneous alcohol injection), external beam radiation and systemic therapies, but surgical resection remains the only potentially curative treatment modality (9-12).
LRs should be considered in patients that are not eligible for transplantation not only in patients without chronic hepatic disease but also in HCC patients with cirrhosis if hepatic function is expected to tolerate partial parenchymal resection (13). Although the best oncological results from LR seem to come from the resection of small tumors, tumor size (even for those lesions larger than 10 cm) is not any longer indicative of resectability (14). Despite the fact that large HCC are associated with high incidence of vascular invasion, even large and locally advanced HCC with portal involvement seem to benefit from major LR (13,14).
Since 1991 when Reich et al. reported the LLR of benign liver tumors and Gagner et al. performed the first laparoscopic complex hepatectomy in 1992, growing experience and development in surgical techniques resulted in widespread application of laparoscopic hepatectomies even to the treatment of HCC (15,16). Nowadays, the indications LLR are almost equal to the open approach (17).
For many years, LLR for HCC were not very popular due to concerns related to tumor seeding, possibility of metastasis to trocar incisions, resection margins and long-term survival (18). However, various meta-analyses demonstrated that LLR for HCC are safe procedures with the potential advantages of lower operative blood loss, lower blood transfusion requirement and lower postoperative hospital length of stay and tumor recurrence compared to open hepatectomies (19,20). In cirrhotic patients, LLR have the further advantages of lower postoperative ascites formation and risk of liver failure (21).
We presented a case that would usually be candidate only for palliative systemic treatment. The patient presented with portal hypertension and cirrhosis, large HCC that would not normally benefit from locoregional therapies, portal involvement and the necessity of major hepatic resection that usually precludes surgical treatment. As a result of adequate preoperative planning, accurate laparoscopic techniques and careful postoperative care, we performed a major LR that resulted in prolonged survival in a case that, otherwise, would probably have limited survival after the initial diagnosis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.10.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50. [Crossref] [PubMed]
- Xiao CZ, Wei W, Guo ZX, et al. A prognosis model for patients with hepatocellular carcinoma and portal vein tumor thrombus following hepatic resection. Oncol Lett 2015;10:2787-94. [Crossref] [PubMed]
- Belli A, Cioffi L, Russo G, et al. Liver resection for hepatocellular carcinoma in patients with portal hypertension: the role of laparoscopy. Hepatobiliary Surg Nutr 2015;4:417-21. [PubMed]
- Cherqui D, Laurent A, Tayar C, et al. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg 2006;243:499-506. [Crossref] [PubMed]
- Cheung TT, Lo CM. Laparoscopic liver resection for hepatocellular carcinoma in patients with cirrhosis. Hepatobiliary Surg Nutr 2015;4:406-10. [PubMed]
- Nakahira S, Takeda Y, Katsura Y, et al. Laparoscopic left hepatectomy with tumor thrombectomy in patients with hepatocellular carcinoma concomitant with advanced portal vein tumor thrombus. Surg Endosc 2014;28:3505. [Crossref] [PubMed]
- Surjan RC, Makdissi FF, Basseres T, et al. Laparoscopic right hepatectomy with vascular reconstruction for the treatment of an advanced hepatocellular carcinoma with portal vein tumor thrombosi. Asvide 2018;5:818. Available online: http://www.asvide.com/article/view/27882
- Yeh MM, Yeung RS, Apisarnthanarax S, et al. Multidisciplinary perspective of hepatocellular carcinoma: A Pacific Northwest experience. World J Hepatol 2015;7:1460-83. [Crossref] [PubMed]
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-12. [Crossref] [PubMed]
- Brown DB, Gould JE, Gervais DA, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20:S425-34. [Crossref] [PubMed]
- Chino F, Stephens SJ, Choi SS, et al. The role of external beam radiotherapy in the treatment of hepatocellular cancer. Cancer 2018;124:3476-89. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Korean Liver Cancer Study Group (KLCSG). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Korean J Radiol 2015;16:465-522. [Crossref] [PubMed]
- Lee SG, Hwang S, Jung JP, et al. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg 2007;94:320-6. [Crossref] [PubMed]
- Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc 1992;6:97-8.
- Machairas N, Papaconstantinou D, Stamopoulos P, et al. The Emerging Role of Laparoscopic Liver Resection in the Treatment of Recurrent Hepatocellular Carcinoma: A Systematic Review. Anticancer Res 2018;38:3181-6. [PubMed]
- Cai X. Laparoscopic liver resection: the current status and the future. Hepatobiliary Surg Nutr 2018;7:98-104. [Crossref] [PubMed]
- Lai EC, Tang CN, Ha JP, et al. Laparoscopic liver resection for hepatocellular carcinoma: ten-year experience in a single center. Arch Surg 2009;144:143-7. [Crossref] [PubMed]
- Li N, Wu YR, Wu B, et al. Surgical and oncologic outcomes following laparoscopic versus open liver resection for hepatocellular carcinoma: A meta-analysis. Hepatol Res 2012;42:51-9. [Crossref] [PubMed]
- Xiong JJ, Altaf K, Javed MA, et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol 2012;18:6657-68. [Crossref] [PubMed]
- Belli G, Fantini C, D'Agostino A, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc 2007;21:2004-11. [Crossref] [PubMed]
Cite this article as: Surjan RC, Makdissi FF, Basseres T, Machado MAC. Advanced hepatocellular carcinoma with portal tumor thrombosis in cirrhotic patient: laparoscopic right hepatectomy with vascular reconstruction. J Vis Surg 2018;4:223.