
How to do: technique of liver hanging maneuver—step by step
Introduction
In 1952, Lortat-Jacob and Robert (1) firstly had described the complete liver mobilization and extrahepatic vascular control for right hepatectomy. This technique was adopted by most hepatic surgeons, becoming the conventional approach to right resection, because it increased the safety of major hepatectomies (2). In 1992 Ozawa proposed the anterior approach (AA) to circumvent to some problems of liver mobilization: (I) high risk of hemorrhage in difficult right-sided mobilization (especially in huge tumors invading the surrounding structures) (3); (II) intraoperative systemic hemodynamic instability due to twisting and compression of inferior vena cava (IVC) (4); (III) risk of tumor rupture or systemic dissemination of cancer cells due to manipulation of tumor (5); (IV) damage of the remnant liver caused by mechanical compression of parenchyma and inflow reduction due to pedicle torsion (4,6). The AA consists of parenchyma transection without prior mobilization of the liver. The main advantages consist in: decreased intraoperative blood loss and transfusion requirements (3,4); these benefits may be explicated because first of all the mobilization of right liver is easier after parenchymal transection, even with large tumors and secondly there is minimal manipulation of the tumor (no-touch technique). Moreover, the AA contribute to a better preservation of post-operative liver function by avoiding pedicle torsion and mechanical compression of the remnant liver (6,7).
However, the principal disadvantage of the AA is due to perform an optimal bleeding control in the deeper parenchymal plane as a result of the difficulty involved in elevating the liver and providing effective hemostatic manual compression (3,8).
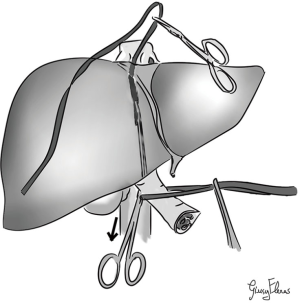
In 2001, Belghiti et al. (9) described a technique called “liver hanging maneuver” (LHM) (Figure 1): consisting in lifting the liver during parenchymal transection by a tape passed between the anterior surface of IVC and the liver. In this manner surgeons achieve the effective vascular control especially for the deeper parenchymal transection, so they can perform an AA easier and safer. Moreover, LHM gives another important advantage consisting in guidance of the direction of anatomic parenchymal transection (9,10).
The avascular plane of RHIVC: anatomic basis (Figure 2)
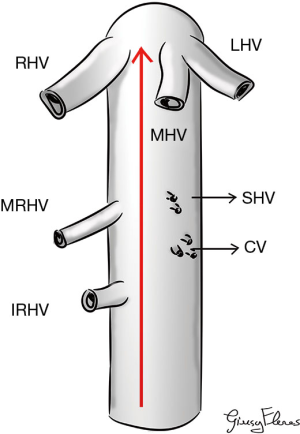
LHM can be performed due to the existence of a longitudinal avascular and virtual space between anteriorly the posterior face of liver and posteriorly the anterior face of IVC. In fact, the most important and risky point is the dissection along the anterior wall of the retrohepatic IVC (RHIVC) (9). The avascular plane of RHIVC is located at 10–11 o’ clock along the anterior face of IVC; it is firstly described by Couinaud in 1981 (11). This avascular plane was defined as the smallest distance between 2 veins on the clamp progression during dissection, this distance was measured in 8.7±2.3 mm (2–15 mm) by Trotovsek et al. (12).
Even if the anatomy of RHIVC and its venous tributaries are variable and complex, anyway RHIVC presents some constant features (13).
In particular we can summarize:
- Main hepatic veins: right hepatic vein (RHV), common trunk joining the middle hepatic vein (MHV) and the left hepatic vein (LHV);
- Left anterolateral part of the RHIVC receives the caudate processus vein (CV) in middle position;
- Right anterolateral part of the RHIVC receives the CV in its caudal part;
- Right middle hepatic vein (MRHV) and/or right inferior hepatic vein (IRHV) in its cranial part. MRHV mainly receives venous drainage of segment 7, it is often unique. IRHV most frequently receives the venous drainage of segment 6 and sometimes it can be an accessory of segment 7;
- Short hepatic veins (SHVs) are variable in position, dimension and number; they receive the venous drainage of segment 8 and the majority are submillimetric;
- In 7–15% of cases small SHVs can be found in this avascular plane (14-16).
Our LHM (technical variant)—step by step (Figure 3)

This method is used routinely by the Reims Team and it is similar to the one described by Belghiti et al. (9) in 2001 with some peculiarities: it is a combined bidirectional up and down (or cranio-caudal) approach.
This approach is adopted to facilitate the right hepatectomy with AA, but it can be modified for left hepatectomy.
First of all we expose the bifurcation of the RHV, MHV and LHV and the anterior surface of the IVC. We prepare the space between the RHV and MHV by separating them with a curved dissector for 3–4 cm. With very smooth and gently movements to downward we expose the plane between IVC and liver capsule.
After that we open the space between the IVC and the infra-hepatic caudate process to expose the anterior face of RHIVC. Normally, it can be found a retro-hepatic vein from segment 6 (IRHV), we usually isolated and sectioned this vein, because it can be hindered during the LHM causing a complicated bleeding. From the top, we continue the gently hydro-dissection of the anterior plane of RHIVC at 10–11 o’ clock along the anterior face of RHIVC between the RHV and MHV. From down to up, along the avascular plane of RHIVC we introduce a slightly curved aortic clamp behind the caudate lobe and it passed cranially along the anterior surface of IVC towards the space prepared between 10 and 11 o’ clock position and between the previously dissected space RHV and MHV. Completed the dissection, the hepatic parenchyma is suspended with a 14 Fr nasogastric tube, therefore this traction allows to control every time eventually venous bleeding and to recognize the bleeding vessel. Moreover, we use the nasogastric tube as a gently dissector to complete the atraumatic preparation of the avascular space due to its rigidity.
We have adopted a gently dissection of the avascular space using: (I) smooth and gently movements; (II) hydro-dissection; (III) 14 Fr nasogastric tube.
Discussion
In 2001 Belghiti et al. (9) firstly have developed LHM for right hepatectomy, making the AA easier and safer, after that LHM was modified for left hepatectomy, living donor hepatectomy and for a superior exposure of the suprahepatic region during hepatectomy in orthotopic liver transplantation with IVC preservation (13).
Among the important advantages of this technique we include that the nasogastric tube guides the surgeon along the correct anatomical dissection plane, facilitating the parenchymal transection along the shortest route. In particular, it allows to maintain the optimal orientation in those conditions in which the anatomy is highly distorted, such as the segmental atrophy of the liver, large tumors, polycystic disease and in the embolization of the portal vein.
With the upward traction of the nasogastric tube the surgeon can obtain both a reduction of the venous backflow bleeding (such as a digital compression), allowing a bloodless transection, and the opening of the dissection and transection plane thus being able to more easily identify and manage the bile ducts and vessels, even in the deeper planes (10,18,19).
Moreover, surgeons can achieve a complete vascular exclusion of the liver along the transection plane performing a LHM combined with a Pringle’s maneuver.
Recently, our group have described a combined technique called: “double-tightened maneuver” (20).
This modified double LHM lead to total vascular exclusion of the liver parenchymal transection area and to permanent control of the outflow from segment 4, 5 and 8 during hepatectomy.
Thus, we perform a total occlusion of the outflow during liver transection, by two lateral tapes tightened with tourniquets.
In particular, as we have reported: the left tape is passed between the right and left Glisson’s pedicles while the right tape is passed under the right hepatic artery to be on the right side of the right Glisson’s pedicle. The lateral two tapes can be gradually tightened and clamped by a flexible tourniquet, so obtaining an interruption of the right portal flow while maintenance the left portal flow associated to a total exclusion of blood flow from the parenchymal transection area.
In addition, we put a third tape (we prefer a nasogastric tube 14 Fr) (20) to facilitate the better exposure and hemostasis of the lower parenchymal plane above the IVC, as the classic technique of the LHM.
This combined technique preserve the remnant liver from ischemic insult, limiting the vascular exclusion to the transection parenchyma line. The total inflow and outflow control is obtained in an easier and less traumatic way compared to the classic total vascular exclusion technique (21-23).
In literature the reported vascular injury risk during LHM vary about 85–93%, for this reason in 7–15% of cases this space can be vascularized by a lower density of veins (12,14-16,24).
The possibility rate to perform LHV is between 96–100% in recent 5 studies, the inability to complete the LHV with suspension of the liver was related to the absence of interhepatocaval space due to tumor infiltration, inflammatory or post-operative strict adhesions between liver and the retro-hepatic IVC, and/or enlarged liver. Anatomic vascular variations has never hindered the LHM (9,10,24-26).
This technique is not burdened by mortality, minor accidents are uncommon (0–7%) and are always of vascular nature (10,18). In fact, during LHM the main cause of bleeding is the dissection into the subcapsular plane, because it may begin in a too superficial plan (10,18). Therefore to avoid this bleeding, as we have shown in our video, it is essential to start the dissection between RHV and MHV, proceeding in to incision and dissection of all the fibrous tissue in front of IVC.
Moreover, it is crucial to expose completely the anterior wall of IVC by incising all the covering fibrous tissue. In most cases to apply in safety the LHM it is crucial to perform the best caval exposure associated or not with the entire cranio-caudal dissection.
As we shown in our video, we perform both the opening of all the covering fibrous tissue in front of IVC and we push to the maximum the cranio and caudal dissection even blinded with a smooth and gently hydro-dissection.
Furthermore, the injury of an SHV can be a cause of bleeding, it can be explained by an error in dissection plane (i.e., wrong direction—too pushed to the left or to the right of the IVC), or rarely due an aberrant SHV in avascular plane (10,13,18). Surgeon can control this complication by putting gauze in the dissection plane and releasing the liver traction. Normally, the liver with his weight provides an enough tamponade to achieve hemostasis, in addition these vessels present a small size and the circular muscular fibers of the junction of the SHVs to RHIVC have a potential contraction. Mobilization of the liver becomes necessary in case of persistent bleeding. Furthermore, a major hepatic vein thrombosis or compression determines a compensatory hypertrophy of the SHVs with the inaccessibility to the avascular plane (considerable reduction or obliteration). Thus, the blind dissection among the avascular plane should be carried with extreme attention (13,18,27).
The infiltration of the avascular plane by liver lesion is the only contraindication (18). Systemic chemotherapy may render the retrohepatic space free of tumor by its regression, permitting into consideration the possibility to use a LHM (18).
Nowadays, those conditions like cirrhosis, preoperative transcatheter arterial chemoembolization and/or portal vein embolization, large tumor size do not significantly compromise the success rate of the LHM (18).
Notably, preoperative triphasic CT-scan can help to evaluate the possibility to perform a LHM: special attention should be carefully given to the state of RHIVC. Therefore LHM can be considered if tumors do not infiltrate the avascular space (18). Moreover, the eventually strict adhesion between IVC and liver by previous surgery or severe tumor inflammation should be carefully evaluated before pursuing a LHM.
Conclusions
The LHM is a useful technique giving a safe AA of major hepatectomies.
We think our video can help surgeons especially junior surgeons to understand better LHM and maybe to reproduce it more facility, reducing any risks correlated to this maneuver.
Surgeons should pay attention performing LHM to avoid major bleeding during blind retrohepatic dissection.
The tumor or inflammation infiltration of the retrohepatic avascular space remains the absolute contraindication for LHM.
If LHM is performed without major bleeding and in the correct avascular plane, it gives important benefits as: less tumor dissemination, reduced hemorrhage, less ischemic damage of remnant liver, improved intraoperative hemodynamic stability, providing vascular outflow control and improve better exposure of deep parenchymal plane during transection.
Acknowledgments
The authors thank Giusy Fleres for having designed the anatomical figures.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.09.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lortat-Jacob JL, Robert HG. Well defined technic for right hepatectomy. Presse Med 1952;60:549-51. [PubMed]
- Belghiti J, Dugué L. Technique for right hepatectomy. J Chir (Paris) 1998;135:19-22. [PubMed]
- Liu CL, Fan ST, Lo CM, et al. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg 2000;232:25-31. [Crossref] [PubMed]
- Lai EC, Fan ST, Lo CM, et al. Anterior approach for difficult major right hepatectomy. World J Surg 1996;20:314-7; discussion 318. [Crossref] [PubMed]
- Louha M, Nicolet J, Zylberberg H, et al. Liver resection and needle liver biopsy cause hematogenous dissemination of liver cells. Hepatology 1999;29:879-82. [Crossref] [PubMed]
- Ozawa K. Hepatic function and liver resection. J Gastroenterol Hepatol 1990;5:296-309. [Crossref] [PubMed]
- Ozawa K. Liver surgery approached through the mitochondria : the Redox theory in evolution. Karger, 1992:222.
- Liu CL, Fan ST, Cheung ST, et al. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg 2006;244:194-203. [Crossref] [PubMed]
- Belghiti J, Guevara OA, Noun R, et al. Liver hanging maneuver: A safe approach to right hepatectomy without liver mobilization. J Am Coll Surg 2001;193:109-11. [Crossref] [PubMed]
- Kokudo N, Sugawara Y, Imamura H, et al. Sling suspension of the liver in donor operation: a gradual tape-repositioning technique. Transplantation 2003;76:803-7. [Crossref] [PubMed]
- Sutherland F, Harris J. Claude Couinaud: a passion for the liver. Arch Surg 2002;137:1305-10. [Crossref] [PubMed]
- Trotovsek B, Belghiti J, Gadzijev EM, et al. Anatomical basis of the liver hanging maneuver. Hepatogastroenterology 2005;52:728-30. [PubMed]
- Gaujoux S, Douard R, Ettorre GM, et al. Liver hanging maneuver: an anatomic and clinical review. Am J Surg 2007;193:488-92. [Crossref] [PubMed]
- Hirai I, Murakami G, Kimura W, et al. How should we treat short hepatic veins and paracaval branches in anterior hepatectomy using the hanging maneuver without mobilization of the liver? An anatomical and experimental study. Clin Anat 2003;16:224-32. [Crossref] [PubMed]
- Sato TJ, Hirai I, Murakami G, et al. An anatomical study of short hepatic veins, with special reference to delineation of the caudate lobe for hanging maneuver of the liver without the usual mobilization. J Hepatobiliary Pancreat Surg 2002;9:55-60. [Crossref] [PubMed]
- Meng WCS, Shao CX, Mak KL, et al. Anatomical justification of Belghiti’s “liver hanging manoeuvre” in right hepatectomy with anterior approach. ANZ J Surg 2003;73:407-9. [Crossref] [PubMed]
- Fleres F, Piardi T, Sommacale D. Liver hanging maneuver (technical variant)-step by step. Asvide 2018;5:807. Available online: http://www.asvide.com/article/view/27663
- Ogata S, Belghiti J, Varma D, et al. Two hundred liver hanging maneuvers for major hepatectomy: A single-center experience. Ann Surg 2007;245:31-5. [Crossref] [PubMed]
- Tanaka H, Takemura S, Ohba K, et al. Convenience of a tape-guiding technique in different types of hepatectomy. Hepatogastroenterology 2008;55:160-3. [PubMed]
- Piardi T, Chetboun M, Cavallari S, et al. Liver Double-Tightened Maneuver: Optimal Outflow Control During Liver Parenchymal Transection of the Right and Left Hepatectomies. J Am Coll Surg 2017;224:e11-6. [Crossref] [PubMed]
- Abdalla EK, Noun R, Belghiti J. Hepatic vascular occlusion: which technique? Surg Clin North Am 2004;84:563-85. [Crossref] [PubMed]
- van Gulik TM, de Graaf W, Dinant S, et al. Vascular Occlusion Techniques during Liver Resection. Dig Surg 2007;24:274-81. [Crossref] [PubMed]
- Gurusamy K, Kumar Y, Sharma D, et al. Methods of vascular occlusion for elective liver resections. In: Gurusamy KS. editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd, 2007:CD006409.
- Ettorre GM, Douard R, Corazza V, et al. Anatomical basis of liver hanging maneuver: a clinical and anatomical in vivo study. Am Surg 2007;73:1193-6. [PubMed]
- Ettorre GM, Vennarecci G, Boschetto A, et al. Feasibility of hanging maneuvers in orthotopic liver transplantation with inferior vena cava preservation and in liver surgery. J Hepatobiliary Pancreat Surg 2004;11:155-8. [Crossref] [PubMed]
- Suzuki M, Unno M, Katayose Y, et al. Hepatic resection through an anterior approach employing a modified liver hanging maneuver in patients with a massive liver tumor severely oppressing the inferior vena cava. Hepatogastroenterology 2004;51:1459-63. [PubMed]
- Kim SH, Park SJ, Lee S, et al. Various Liver Resections Using Hanging Maneuver by Three Glisson’s Pedicles and Three Hepatic Veins. Ann Surg 2007;245:201-5. [Crossref] [PubMed]
Cite this article as: Fleres F, Piardi T, Sommacale D. How to do: technique of liver hanging maneuver—step by step. J Vis Surg 2018;4:213.


