
Planning minimally invasive mitral valve surgery
Introduction
Although minimally invasive mitral valve surgery (MIMVS) was already described in the late 1990’s (1,2), adoption rate of this technique remains rather low >20 years later (15% of all mitral procedures in the United States) and MIMVS is not mentioned in most recent valvular heart disease guidelines (3-6). This can partly be explained by the accompanying increased technical difficulties in MIMVS with a steep learning curve (7), potentially holding interested surgeons back in starting or continuing such a comprehensive program (8). Additionally, there is still a lack of convincing data favoring this technique, with only a few inconclusive randomized controlled trials and limited meta-analyses of retrospective studies dealing with this subject (9-11). In general, most of these comparative studies demonstrate less bleeding, less wound infections, more rapid detubation and shorter intensive care unit and hospital stay in spite of longer cardiopulmonary bypass (CPB) and clamping times with an increased risk of stroke. Echocardiographic follow-up of these patients revealed no difference in residual mitral regurgitation (MR).
However, access (12) (different locations and sizes of incisions), vision (13) (direct, video-assisted or totally endoscopic) and CPB cannulation strategies (14) (antegrade vs. retrograde) vary between centers and make these results even more difficult to compare and interpret.
In our philosophy, precise patient selection and extensive pre-operative planning is imperative for this procedure and could lead to a reduction in complications following MIMVS, potentially resulting in superiority of MIMVS over conventional surgery through a median sternotomy. Therefore, in our institution, all patients considered for MIMVS undergo a standard preoperative diagnostic pathway evaluated by a dedicated mitral valve heart team after which they are allocated to their designated treatment (Figure 1). In this philosophy of Medicine, we select the patients that benefit most of our standardized technique, rather than adapting the surgical technique to the specific patient (16).
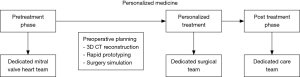
The current review aims to present an overview of current literature on this topic together with our institutions experience in this field as a guidance for surgeons starting this program, in order avoid potential preventable complications.
Standardized preoperative diagnostic pathway
All patients referred for surgical correction of mitral valve disease are discussed in our weekly convening dedicated mitral valve heart team. If a patient is considered eligible for a minimally invasive approach (i.e., no need for concomitant coronary artery bypass grafting, aortic valve replacement etc.), the patients undergoes a standardizes preoperative diagnostic pathway consisting of the following modalities.
Electrocardiography
All current and past electrocardiographic examinations are reviewed for occurrences of atrial fibrillation (AF) and ventricular dyssynchrony. In case of a reported episode of AF, the patient is discussed in our dedicated rhythm heart team (consisting of rhythm surgeons and electrophysiologists). According to most recent guidelines, patients with paroxysmal, persistent and long-standing persistent AF have a class I indication for concomitant surgical ablation and are planned for a concomitant procedure (17).
Chest X-ray
Standard performed chest X-rays are assessed for deviant thoracic anatomy and height of the right hemi diaphragm. Additionally, acute and chronic pulmonary pathology can be identified using this simple imaging modality, potentially influencing the preoperative planning pathway.
Echocardiography
Before referral to our institution, patients undergo standard transthoracic echocardiography (TTE) at their own hospital, which is assessed by imaging specialists of our mitral valve heart team. As most important limitation, this technique is highly operator- and interpreter-dependent, warranting assessment by imaging cardiologists with (echocardiographic) expertise in valvular heart disease. As an advantage, TTE is widely available, cost-effective and has excellent results for functional assessment (18).
For evaluation of severity of mitral valve disease, TTE remains the gold standard. Using TTE, MR severity is evaluated by valve morphology, colour flow jet, vena contracta width, pulmonary vein flow, time-velocity integral of mitral inflow, effective orifice regurgitant orifice area and regurgitant volume (19). However, regarding colour flow jet, one should be careful to base assessment of severity solely on the extent of the jet. Although a large jet reaching the pulmonary veins is usually considered severe, small jets reaching just above the mitral valve can be misleadingly interpreted as non-severe but can potentially be influenced by increased left atrial pressure (20). Additionally, young patients with extensive myxomatous valve disease (Barlow’s) can exhibit all signs of severe MR, but due to mitral annular disjunction, the valve itself can be fully displaced into the atrium without a large colour flow jet (21). Therefore, severity assessment should be determined using an integrative approach including all (if available) aforementioned qualitative, semi-quantitative and quantitative parameters. In addition to severity of MR, TTE gives a general overview of cardiac function and other valvular pathologies. In relation to the mitral valve, it is imperative to evaluate pulmonary artery pressure, right ventricular function and potential concomitant tricuspid regurgitation (TR). Although right ventricular function is not evaluated by most risk scores, right ventricular dysfunction has proven to be an under-recognized predictor of mortality after cardiac surgery (22). Furthermore, thresholds for concomitant tricuspid repair in mitral valve surgery have lowered over the past years and concomitant repair has been associated with improved outcomes (23). According to recent guidelines, additional tricuspid repair should be considered in case of tricuspid annular dilatation >40 mm, even regardless of severity of TR.
In case of degenerative MR with prolapsing segment(s), repair is likely, warranted and associated with lower morbidity, mortality and improved survival compared to mitral valve replacement (24-26). For functional MR, echocardiographic parameters can help to determine probability of a successful repair. The following unfavorable echocardiographic parameters have been described; annular dilatation >50 mm, involvement of >3 leaflet scallops including the anterior mitral valve leaflet and severe mitral annular calcification (27). Furthermore, Carpentier functional type IIIa (rheumatic) valves and ischemic valves (Carpentier type IIIb) with severe ventricular dilatation have a low repair probability (the latter due to postoperative ventricular remodeling).
If repair is likely, as in degenerative disease, patients in our institution undergo subsequent transesophageal echocardiography (TEE), as it is superior to TTE for localization of mitral prolapse (28), to determine the exact mechanism of regurgitation (i.e., specific prolapsing segments) in order to predict procedural repair strategy. Using TEE, precise measurement of the anterior mitral leaflet length and intercommissural distance can aid to predict the required ring size for mitral repair (29).
In complex degenerative pathology, three-dimensional (3D) reconstruction of the diseased valve can be created using dedicated software (15), allowing the operator to assess the valve from all angles (Figure 2). Additionally, these computer aided designs are superior in ring size prediction (29). For specific cases, these reconstructions can even be used to 3D print (rapid prototyping) the patient specific pathological valve, which is discussed in another chapter of this issue.

Coronary angiography
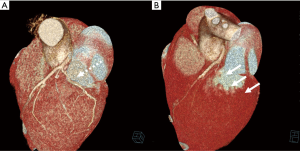
As for all non-coronary cardiac procedures, coronary angiography (CAG) is paramount for evaluation of potential subclinical coronary artery disease (CAD) requiring concomitant coronary artery bypass grafting. In addition to evaluation of CAD, CAG can also be used for planning of mitral valve surgery. First, mitral annular calcification can be assessed in location and extent, potentially influencing procedural strategy. Furthermore, and most importantly, the trajectory of the circumflex artery (RCx) can be determined in relation to the mitral valve. A less well-known, uncommon, but disastrous complication in mitral valve surgery is accidental (partial) occlusion of the RCx (31,32). It is even thought to be an under-recognized and under-reported complication, as many patients present with ischemia-like features and reduced ventricular function directly after mitral valve surgery (33). This complication can be the result of either (I) complete obliteration of the RCx lumen by annular sutures or (II) by kinking of the RCx towards the mitral valve due to traction of annular sutures (Figures 3,4). Retrospectively, this complication was observed most in patients with a left dominant coronary system (35). This can be explained by the course of the RCx, which runs closer to the mitral valve in hearts with left dominance (36). Coronary angiography should be used as a guidance in procedural strategy in patients with left dominance, as left dominance is not a contraindication for MIMVS or conventional mitral valve surgery, rather a warning. Care should be taken in placement of annular sutures around the anterolateral commissure, as post-mortem studies revealed this to be the area with the shortest distance to the RCx, corresponding to the proximal one-third of the coronary artery (35).

Computed tomography (CT) and anatomical reconstructions
Indication for surgical approach (i.e., minithoracotomy or sternotomy) is based on anatomical features and comorbidities. In order to assess anatomical eligibility, standard contrast-enhanced CT scans are performed at our institution in all patients in work-up prior to mitral valve interventions. CT is a relatively low-cost non-invasive diagnostic imaging modality which produces computer-processed combinations of a predefined number of X-rays. An important downside of this modality is the use of radiation and its subsequent attributable cancer risk (37). With recent technological advancements, such as automated tube voltage selection, tube current modulation and ultra-fast scan acquisition, radiation dose can be reduced markedly (38,39). Combined with a reduction of contrast media volume used, decreasing prevalence of contrast induced neuropathy, CT has become more widely available and accepted (40). As described previously, these CT images can then be used to acquire a 3D reconstruction of the patients’ anatomy (Vesalius 3D, PS-Medtech, Amsterdam, the Netherlands) (41).
The first step in realising these high-quality, reliable and reproducible reconstructions of patients’ anatomy prior to surgery is the initial image acquisition. Based on our early experience and different acquisition protocols, nowadays an electrocardiography (ECG)-triggered helical CT angiography of the aortic root and ascending aorta followed by a high-pitch spiral CT angiography from the aortic arch to the femoral bifurcation is performed in all patients. Close collaboration with the local department of radiology is imperative to develop the ideal scan protocol. Using dedicated software, the 3D reconstruction is then derived from these high-quality initial scans (41,42).
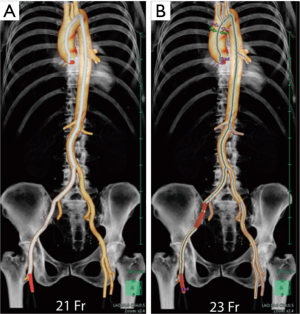
First, suitability for CPB cannulation and safe retrograde perfusion is evaluated on these reconstructions. MIMVS using retrograde perfusion has been associated with increased risk of stroke (43). However, in our vision, risk of stroke can be minimalized by preoperative assessment of the abdominal aorta and peripheral vessels (Figure 5). A recent study by our research group demonstrated approximately 30% of patients to be less suitable for a minimally invasive approach, as we found the following characteristics on CT reconstruction: severe calcification of the abdominal aorta (porcelain aorta), iliofemoral vessel tortuosity, (ascending) aortic elongation and extensive pericardial calcification (41). These patients were excluded from a minimally invasive approach, as a sternotomy with antegrade perfusion would be safer, avoiding the risk of stroke. Additionally, in severely elongated aortas, we try to avoid aortic occlusion with the trans-thoracic clamp, while we demonstrated aortic elongation to be age-dependent and a risk factor for the development of type A aortic dissection (45,46).

Of note, one could argue to tailor surgical and perfusion techniques to the individual patient based on CT, as different centers have demonstrated excellent results with antegrade perfusion techniques in MIMVS using axillary artery cannulation or direct aortic cannulation (14,47,48). However, we advocate a standardized approach in all patients undergoing MIMVS, rather than using numerous different strategies.
Second, we determine the strategy for aortic occlusion. Our preferred method for aortic clamping is by use of an endo-aortic balloon (IntraClude, Edwards Lifesciences, Irvine, CA, USA) as it has similar outcome compared to the transthoracic aortic clamp (Chitwood Clamp, Scanlan International, St Paul, MN, USA) and does not require placement of an aortic needle for cardioplegia delivery while this is integrated in the system, making it especially ideal for reoperative mitral valve surgery (49,50). Ascending aortic dilatation is evaluated on CT, as the endo-aortic balloon requires an aortic diameter <4 cm to safely achieve secure occlusion (51). Furthermore, the balloon is introduced in a special side port of the femoral arterial cannula and advanced under TEE guidance to its position in the ascending aorta. To facilitate sufficient lumen for introduction of the balloon, a minimal cannula size of 19 Ch is required. We determine our cannula size and cannulation side prior to the procedure using dedicated software, which simulates the introduction and advancement of the cannula using iliofemoral vessel diameter calculations (Figure 6, Synapse3D, Fujifilm, Tokyo, Japan).
Third, the ideal intercostal space for the incision of the mini-thoracotomy is determined preoperatively, based on the level of the left atrium, Waterston’s groove and the mitral valve in relation to the height of the right hemi-diaphragm (52) (Figure 7). Figure 8 demonstrates an example of a patient with excellent anatomical features for a minimally invasive approach.


As an additional merit, CT can be used for evaluation of coronary artery disease in young low-risk patients. Of note, coronary CT can only be used to rule out coronary disease (55). If CT shows no coronary calcifications, coronary angiography is not required. In case of some calcification, CT is still insufficient to quantify stenoses, and additional invasive angiography is warranted (56).
Finally, we observed numerous incidental findings (incidentalomas) on CT. In our clinical practice, CT reveals in approximately 25% of all patients a subclinical incidentaloma, of which one-third are actual malignancies requiring follow-up or intervention, sometimes prior to mitral valve surgery.
Future perspectives
With the emergence of minimally invasive and even off-pump beating-heart procedures, patient selection and preoperative planning will play an increasingly important role in the near future. Most of these off-pump procedures, such as trans-catheter edge-to-edge mitral valve repair (57), trans-catheter (in)direct annuloplasty (58,59) and surgical beating-heart mitral valve repair (60-62) require intra-procedural TEE-guidance.
First, preoperative TEE is warranted in these patients, as it is imperative to evaluate the possibility to obtain images of sufficient quality as during the actual procedure. Second, TEE is used to identify suitable patients with eligible valvular pathology for such an approach as described in this review.
Regarding CT, recent advancements in reduction of radiation dosages and contrast media volume will result in an increased use of this imaging modality, making it more available as risk of complications decreases. Currently, cardiac CT is not well-established enough to determine the exact mechanism of valvular dysfunction compared to echocardiography and quantification of coronary artery disease compared to invasive angiography. However, we foresee important developments in these areas, making invasive coronary angiography redundant as it carries a higher complication rate (63). An important additional merit of CT over conventional echocardiography is that CT can be used to precisely define and measure mitral annular geometry (64,65) which can facilitate a preoperative prediction of the ring size for surgical or trans-catheter mitral valve repair. Finally, with recent developments in trans-catheter mitral valve replacement technologies (66,67), preoperative assessment of the left ventricular outflow tract (LVOT) and prediction of the neo-LVOT will become mandatory in patient selection for these procedures, promoting the use of CT in these patients (68).
Conclusions
As mitral valve procedures are becoming less invasive, patient selection and preoperative planning are becoming increasingly important. Using different imaging modalities as CT and echocardiography, precise patient selection could lead to a reduction of complications, as high-risk patients for these operations can be filtered out preoperatively, potentially resulting in superiority of MIMVS over conventional surgical approaches.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Minimally Invasive Mitral Valve Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.09.07). The series “Minimally Invasive Mitral Valve Surgery” was commissioned by the editorial office without any funding or sponsorship. PSN served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mohr FW, Onnasch JF, Falk V, et al. The evolution of minimally invasive valve surgery--2 year experience. Eur J Cardiothorac Surg 1999;15:233-8; discussion 238-9. [Crossref] [PubMed]
- Carpentier A, Loulmet D, Aupecle B, et al. C R Acad Sci III 1998;321:437-42. [Computer assisted open heart surgery First case operated on with success]. [Crossref] [PubMed]
- Gammie JS, Zhao Y, Peterson ED, et al. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2010;90:1401-8, 10.e1; discussion 1408-10.
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-132. [Crossref] [PubMed]
- Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91. [Crossref] [PubMed]
- Loulmet DF, Carpentier A, Cho PW, et al. Less invasive techniques for mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:772-9. [Crossref] [PubMed]
- Dogan S, Aybek T, Risteski PS, et al. Minimally invasive port access versus conventional mitral valve surgery: prospective randomized study. Ann Thorac Surg 2005;79:492-8. [Crossref] [PubMed]
- Sundermann SH, Sromicki J, Rodriguez Cetina Biefer H, et al. Mitral valve surgery: right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2014;148:1989-95.e4. [Crossref] [PubMed]
- Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52. [Crossref] [PubMed]
- Little S, Flynn M, Pettersson GB, et al. Revisiting the dome approach for partial sternotomy/minimally invasive mitral valve surgery. Ann Thorac Surg 2009;87:694-7. [Crossref] [PubMed]
- Felger JE, Chitwood WR Jr, Nifong LW, et al. Evolution of mitral valve surgery: toward a totally endoscopic approach. Ann Thorac Surg 2001;72:1203-8; discussion 1208-9. [Crossref] [PubMed]
- Murzi M, Cerillo AG, Miceli A, et al. Antegrade and retrograde arterial perfusion strategy in minimally invasive mitral-valve surgery: a propensity score analysis on 1280 patients. Eur J Cardiothorac Surg 2013;43:e167-72. [Crossref] [PubMed]
- Sardari Nia P, Heuts S, Daemen J, et al. Preoperative planning with three-dimensional reconstruction of patient's anatomy, rapid prototyping and simulation for endoscopic mitral valve repair. Interact Cardiovasc Thorac Surg 2017;24:163-8. [PubMed]
- Loor G, Roselli EE. Imaging and minimally invasive aortic valve replacement. Ann Cardiothorac Surg 2015;4:62-6. [PubMed]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1-160. [Crossref] [PubMed]
- Sugeng L, Mor-Avi V, Weinert L, et al. Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation 2006;114:654-61. [Crossref] [PubMed]
- Lancellotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-44. [Crossref] [PubMed]
- McCully RB, Enriquez-Sarano M, Tajik AJ, et al. Overestimation of severity of ischemic/functional mitral regurgitation by color Doppler jet area. Am J Cardiol 1994;74:790-3. [Crossref] [PubMed]
- Enriquez-Sarano M. Mitral Annular Disjunction: The Forgotten Component of Myxomatous Mitral Valve Disease. JACC Cardiovasc Imaging 2017;10:1434-6. [Crossref] [PubMed]
- Haddad F, Denault AY, Couture P, et al. Right ventricular myocardial performance index predicts perioperative mortality or circulatory failure in high-risk valvular surgery. J Am Soc Echocardiogr 2007;20:1065-72. [Crossref] [PubMed]
- Chikwe J, Itagaki S, Anyanwu A, et al. Impact of Concomitant Tricuspid Annuloplasty on Tricuspid Regurgitation, Right Ventricular Function, and Pulmonary Artery Hypertension After Repair of Mitral Valve Prolapse. J Am Coll Cardiol 2015;65:1931-8. [Crossref] [PubMed]
- Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J 2010;31:1958-66. [Crossref] [PubMed]
- Akins CW, Hilgenberg AD, Buckley MJ, et al. Mitral valve reconstruction versus replacement for degenerative or ischemic mitral regurgitation. Ann Thorac Surg 1994;58:668-75; discussion 675-6. [Crossref] [PubMed]
- Gillinov AM, Blackstone EH, Nowicki ER, et al. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg 2008;135:885-93, 93.e1-2.
- Omran AS, Woo A, David TE, et al. Intraoperative transesophageal echocardiography accurately predicts mitral valve anatomy and suitability for repair. J Am Soc Echocardiogr 2002;15:950-7. [Crossref] [PubMed]
- Chandra S, Salgo IS, Sugeng L, et al. Characterization of degenerative mitral valve disease using morphologic analysis of real-time three-dimensional echocardiographic images: objective insight into complexity and planning of mitral valve repair. Circ Cardiovasc Imaging 2011;4:24-32. [Crossref] [PubMed]
- Ender J, Eibel S, Mukherjee C, et al. Prediction of the annuloplasty ring size in patients undergoing mitral valve repair using real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr 2011;12:445-53. [PubMed]
- Heuts S, Olsthoorn JR, Maessen JG, et al. Three-dimensional mitral valve reconstruction based on TEE images. Asvide 2018;5:802. Available online: http://www.asvide.com/article/view/27630
- Hiltrop N, Bennett J, Desmet W. Circumflex coronary artery injury after mitral valve surgery: A report of four cases and comprehensive review of the literature. Catheter Cardiovasc Interv 2017;89:78-92. [Crossref] [PubMed]
- Tavilla G, Pacini D. Damage to the circumflex coronary artery during mitral valve repair with sliding leaflet technique. Ann Thorac Surg 1998;66:2091-3. [Crossref] [PubMed]
- Coutinho GF, Leite F, Antunes MJ. Circumflex artery injury during mitral valve repair: Not well known, perhaps not so infrequent-lessons learned from a 6-case experience. J Thorac Cardiovasc Surg 2017;154:1613-20. [Crossref] [PubMed]
- Heuts S, Olsthoorn JR, Maessen JG, et al. Direct postoperative invasive coronary angiography after mitral valve repair revealing an iatrogrenic total occlusion of the proximal RCx. Asvide 2018;5:803. Available online: http://www.asvide.com/article/view/27631
- Grande AM, Fiore A, Massetti M, et al. Iatrogenic circumflex coronary lesion in mitral valve surgery: case report and review of the literature. Tex Heart Inst J 2008;35:179-83. [PubMed]
- Virmani R, Chun PK, Parker J, et al. Suture obliteration of the circumflex coronary artery in three patients undergoing mitral valve operation. Role of left dominant or codominant coronary artery. J Thorac Cardiovasc Surg 1982;84:773-8. [PubMed]
- Faletra FF, D'Angeli I, Klersy C, et al. Estimates of lifetime attributable risk of cancer after a single radiation exposure from 64-slice computed tomographic coronary angiography. Heart 2010;96:927-32. [Crossref] [PubMed]
- Kok M, Mihl C, Seehofnerova A, et al. Automated Tube Voltage Selection for Radiation Dose Reduction in CT Angiography Using Different Contrast Media Concentrations and a Constant Iodine Delivery Rate. AJR Am J Roentgenol 2015;205:1332-8. [Crossref] [PubMed]
- Zhang LJ, Zhao YE, Schoepf UJ, et al. Seventy-Peak Kilovoltage High-Pitch Thoracic Aortic CT Angiography without ECG Gating: Evaluation of Image Quality and Radiation Dose. Acad Radiol 2015;22:890-7. [Crossref] [PubMed]
- Kok M, Mihl C, Hendriks BM, et al. Optimizing contrast media application in coronary CT angiography at lower tube voltage: Evaluation in a circulation phantom and sixty patients. Eur J Radiol 2016;85:1068-74. [Crossref] [PubMed]
- Heuts S, Maessen JG, Sardari Nia P. Preoperative planning of left-sided valve surgery with 3D computed tomography reconstruction models: sternotomy or a minimally invasive approach? Interact Cardiovasc Thorac Surg 2016;22:587-93. [Crossref] [PubMed]
- Schreurs R, Heuts S, Natour E, et al. The unstoppable heart: an aortocoronary fistula in coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Grossi EA, Loulmet DF, Schwartz CF, et al. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg 2012;143:S68-70. [Crossref] [PubMed]
- Heuts S, Olsthoorn JR, Maessen JG, et al. Three-dimensional anatomical reconstruction of the abdominal aorta and peripheral vessels revealing extensive calcification and tortuosity of the iliofemoral vessels. Asvide 2018;5:804. Available online: http://www.asvide.com/article/view/27632
- Adriaans BP, Heuts S, Gerretsen S, et al. Aortic elongation part I: the normal aortic ageing process. Heart 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Heuts S, Adriaans BP, Gerretsen S, et al. Aortic elongation part II: the risk of acute type A aortic dissection. Heart 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Glauber M, Murzi M, Solinas M. Central aortic cannulation for minimally invasive mitral valve surgery through right minithoracotomy. Ann Cardiothorac Surg 2013;2:839-40. [PubMed]
- Grossi EA, Loulmet DF, Schwartz CF, et al. Minimally invasive valve surgery with antegrade perfusion strategy is not associated with increased neurologic complications. Ann Thorac Surg 2011;92:1346-9; discussion 1349-50. [Crossref] [PubMed]
- Bentala M, Heuts S, Vos R, et al. Comparing the endo-aortic balloon and the external aortic clamp in minimally invasive mitral valve surgery. Interact Cardiovasc Thorac Surg 2015;21:359-65. [Crossref] [PubMed]
- Vallabhajosyula P, Wallen T, Pulsipher A, et al. Minimally Invasive Port Access Approach for Reoperations on the Mitral Valve. Ann Thorac Surg 2015;100:68-73. [Crossref] [PubMed]
- Czesla M, Mogilansky C, Balan R, et al. Evolution of a minimally invasive mitral valve program. J Vis Surg 2016;2:169. [Crossref] [PubMed]
- Ailawadi G, Agnihotri AK, Mehall JR, et al. Minimally Invasive Mitral Valve Surgery I: Patient Selection, Evaluation, and Planning. Innovations (Phila) 2016;11:243-50. [Crossref] [PubMed]
- Heuts S, Olsthoorn JR, Maessen JG, et al. Three-dimensional anatomical reconstruction of the thoracic anatomy revealing a high right hemi-diaphragm in relation the mitral valve and the left atrium. Asvide 2018;5:805. Available online: http://www.asvide.com/article/view/27633
- Heuts S, Olsthoorn JR, Maessen JG, et al. Three-dimensional anatomical reconstruction of the aorta, peripheral vessels and thoracic anatomy, revealing excellent anatomical eligibility for a minimally invasive approach. Asvide 2018;5:806. Available online: http://www.asvide.com/article/view/27634
- Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724-32. [Crossref] [PubMed]
- Sun Z, Choo GH, Ng KH. Coronary CT angiography: current status and continuing challenges. Br J Radiol 2012;85:495-510. [Crossref] [PubMed]
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406. [Crossref] [PubMed]
- Maisano F, Taramasso M, Nickenig G, et al. Cardioband, a transcatheter surgical-like direct mitral valve annuloplasty system: early results of the feasibility trial. Eur Heart J 2016;37:817-25. [Crossref] [PubMed]
- Schofer J, Siminiak T, Haude M, et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 2009;120:326-33. [Crossref] [PubMed]
- Seeburger J, Rinaldi M, Nielsen SL, et al. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: the TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J Am Coll Cardiol 2014;63:914-9. [Crossref] [PubMed]
- Colli A, Manzan E, Rucinskas K, et al. Acute safety and efficacy of the NeoChord proceduredagger. Interact Cardiovasc Thorac Surg 2015;20:575-80; discussion 580-1. [Crossref] [PubMed]
- Gammie JS, Wilson P, Bartus K, et al. Transapical Beating-Heart Mitral Valve Repair With an Expanded Polytetrafluoroethylene Cordal Implantation Device: Initial Clinical Experience. Circulation 2016;134:189-97. [Crossref] [PubMed]
- Achenbach S. Coronary CT angiography-future directions. Cardiovasc Diagn Ther 2017;7:432-8. [Crossref] [PubMed]
- Naoum C, Blanke P, Cavalcante JL, et al. Cardiac Computed Tomography and Magnetic Resonance Imaging in the Evaluation of Mitral and Tricuspid Valve Disease: Implications for Transcatheter Interventions. Circ Cardiovasc Imaging 2017;10. [PubMed]
- Sundermann SH, Gordic S, Manka R, et al. Computed tomography for planning and postoperative imaging of transvenous mitral annuloplasty: first experience in an animal model. Int J Cardiovasc Imaging 2015;31:135-42. [Crossref] [PubMed]
- Regueiro A, Granada JF, Dagenais F, et al. Transcatheter Mitral Valve Replacement: Insights From Early Clinical Experience and Future Challenges. J Am Coll Cardiol 2017;69:2175-92. [Crossref] [PubMed]
- Guerrero M, Dvir D, Himbert D, et al. Transcatheter Mitral Valve Replacement in Native Mitral Valve Disease With Severe Mitral Annular Calcification: Results From the First Multicenter Global Registry. JACC Cardiovasc Interv 2016;9:1361-71. [Crossref] [PubMed]
- Theriault-Lauzier P, Mylotte D, Dorfmeister M, et al. Quantitative multi-slice computed tomography assessment of the mitral valvular complex for transcatheter mitral valve interventions part 1: systematic measurement methodology and inter-observer variability. EuroIntervention 2016;12:e1011-20. [Crossref] [PubMed]
Cite this article as: Heuts S, Olsthoorn JR, Maessen JG, Sardari Nia P. Planning minimally invasive mitral valve surgery. J Vis Surg 2018;4:212.


