Thoracoscopic right S9+10 segmentectomy
S9+10 segmentectomy can be applied to some cT1aN0 NSCLC or some metastases (Figure 1). It is recognized by most authors as one of the most difficult anatomical segmentectomy when done through a thoracoscopic approach (1-3). Reasons are of two different orders: (I) the highly variable distribution of arteries and veins of the basilar segments and (II) the pyramidal shape of these segments that make identification of the intersegmental plane difficult and its division even more problematic. Preoperative modelisation of bronchovascular anatomy is crucial (4) and the use of intraoperative dye marking or fluorescence is not only helpful but almost compulsory (5).
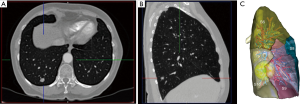
The most difficult part of the procedure is determining the adequate plane between S9+10 and S7+8 and S6. In our early experience, we were stapling the parenchyma from its diaphragmatic aspect to the apex and progressively skewing the direction to the rear in order to spare S6. Eventually, we found safer to separate S6 and S9+10 by creating a tunnel and dividing the plane between S9+10 and S7+8 in a second stage (2). This maneuver is however not so easy and add some complexity to this procedure.
Anatomical landmarks
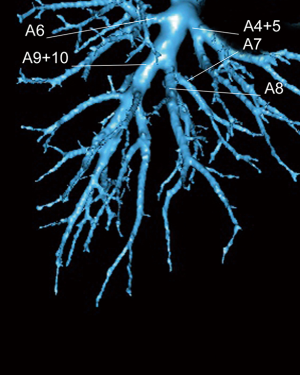
In 90% of the patients, the basilar arterial trunk branches in 2 arteries: A7+8 and A9+10 (Figure 2). All arteries to the lower lobe must be dissected on a sufficient length and clearly identified in order to avoid misidentification. Indeed, in 8% of the cases, there is a common trunk for A8 and A9 (A8+9), with a separate A10 (6). A rare (4% in frequency) but troublesome anatomical variation is the presence of an additional artery rising from A10 or from the truncus intermedius, named A* by Nomori and Okada (6), which is accompanied by a B* bronchus for the so-called sub-superior S* posterior segment (Figure 3).
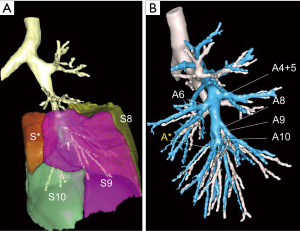
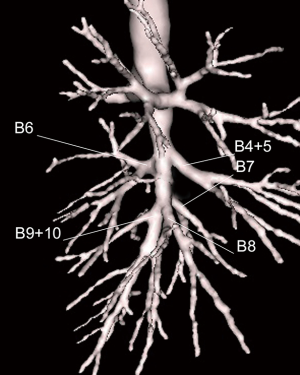
The basilar bronchial trunk usually separates in 2 branches: B7+8 and B9+10 that run posterior to the segmental arteries (Figure 4).
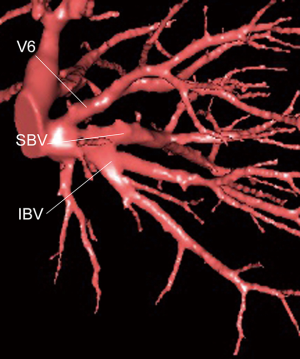
The classical distribution of the inferior pulmonary vein (IPV) into 3 main branches is actually found only in a minority of the patients (6). The inferior basal vein (IBV), which is the lower root of the IPV, does not always represent the venous drainage of segments 9 and 10 (Figure 5). One of its branches can drain S8. It is advisable to control the vein inside the parenchyma, once the segmental bronchus has been divided.
General principles
In our department, anatomic sublobar resections (SLR) are performed for solitary metastases that are not resectable by mere wedge resection, for ground glass opacities, for cT1a primary NSCLC and for some cT1b tumors for patients who have undergone a previous major pulmonary resection and/or with compromised pulmonary function and/or with 2 synchronous or metachronous tumors.
For all complex SLR, and particularly for basilar segmentectomies, a preoperative modelisation is performed, using Visible Patient™ software. It allows for a dynamic navigation in the arteries, veins and bronchi, layer by layer, and calculates the volume of the segments to be resected and a safety margin can be visualized.
Our technique is based on a full thoracoscopic and fissure-based approach, whose principles have been described in details (7). In brief, we operate with a pure monitor display, a high-definition imaging system, a 10 mm deflectable endoscope hold on a robotized scope positioner, 3 to 4 ports without utility incision and a specifically designed instrumentation. Compared to the so-called anterior approach that comprises a dissection of the hilum with a fissure last division, our technique is based on an extensive dissection of the pulmonary artery (PA) branches in the fissure. The segmentectomy is completed with a radical hilar and mediastinal lymph node (LN) dissection, according to a previously reported technique (8) in NSCLC. In summary, stations 2, 4, 7, 8, 9 and 10 on the right side and stations 5, 6, 7, 8, 9 and 10 on the left side are totally removed. All peribronchial (station 11) and interlobar and intersegmental (stations 12 and 13) are cleared. Intersegmental LN and safety margins are examined by frozen section. The procedure is converted to a more extended segmentectomy or even lobectomy in case of invaded LN or insufficient safety margin (1).
As for all right-sided major pulmonary resections, the surgeons stand in the back of the patient (7). Four ports are used: one 12-mm in the mid-axillary line and the 6th intercostal space (ICS) for the deflectable endoscope that is hold on a scope positioner throughout the procedure, two 5-mm ports in the posterior-axillary line for the dissecting instruments and one oval-shape 18 mm × 25 mm in the anterior axillary line, in the 7th ICS.
Dissecting and controlling the arteries
The PA is approached in the middle portion of the major fissure as for a lower lobectomy. Its branches are dissected and A6 is identified. Once this artery has been exposed, the lower lobe is retracted upward and forward to expose the pulmonary ligament.
The pulmonary ligament is incised up to the lower vein using both diathermy and gentle traction on the lower lobe.
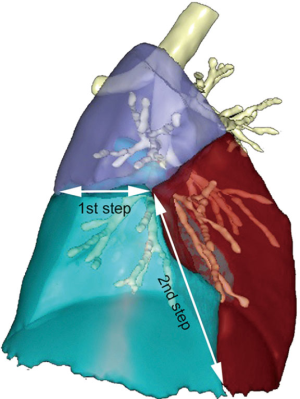
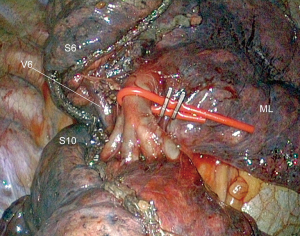
In our previous experience, we were stapling the intersegmental plane from the pulmonary ligament to the hilum, starting from the diaphragmatic side of S9 and S10 and skewing the staple line to the rear when reaching the inferior border of S6. We found this manoeuver approximate and unsatisfactory and now apply a tunneling technique to separate S6 and S9+10, thus facilitating the exposure of arteries (Figure 6). When completed, this maneuver makes vascular dissection easier but it can be tedious, especially in patients with an emphysematous lung that hampers exposure. The procedure is done as follows (Figure 7): the inferior vein is cleared from the surrounding tissues, until the IBV, SBV and V6 are identified. A passage is created between V6 and the IBV (2), using an endo peanut, a blunt tip dissector or a combination of both (Figure 8). The surgeons move then back to the fissure where the bronchovascular elements of S6 have previously been dissected. V6 is identified in the fissure. The tip of the dissector is then inserted below V6 and gently pushed backward until it reaches the defect between V6 and the IBV. A tunnel is thus created and a stapler can be introduced into this tunnel, so that S6 can be pulled apart from S9+10. Continuation of arterial dissection is thus greatly facilitated (Figure 9).

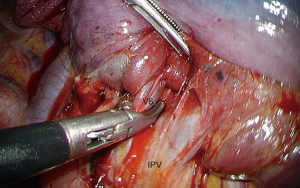
Dissection of arteries is pursued as low as possible and A9+10 is identified and clipped or stapled depending on its diameter (Figure 10). All LNs located at the arterial division must be dissected, removed and sent for frozen section.
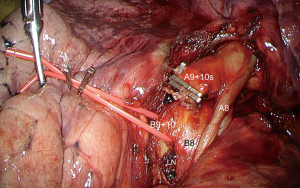
Dissecting and controlling the bronchi
A8 is looped, so that it can be retracted forward or upward to expose the bronchi (Figure 10).
B9+10 is exposed. It is carefully dissected to avoid any tear of the vein running behind it. It is then stapled after a reventilation test.
Dissecting and controlling the veins
Controlling the vein centrally is possible only when IBV, SBV and V6 can be identified and when preoperative modelisation shows that S9 and S10 are drained by the IBV. However, IBV can partly drain S8 and it can be safer to divide only its lowermost tributary, i.e., V10.
In most cases and, especially when the venous anatomy is unclear, it is preferable to control the vein within the parenchyma: the vein appears once the segmental bronchus has been divided and can be severed at this level (Figure 11).
Identifying and dividing the intersegmental plane
We use systemic injection of indocyanine green (ICG) at the dose of 0.2 mg/kg with near-infrared imaging system (Novadaq™) (5). Cautery dots are applied on the demarcation lines, i.e., plan between S8 and S9 and on the diaphragmatic side plan between S9+10 and S8 (Figure 12). This maneuver should be promptly done as the green coloration can vanish rapidly in some patients. The ICG injection can be renewed once. We recommend to start on the diaphragmatic side which is the most difficult to identify. Marking can also be done by intrabronchial injection of methylene blue. Some authors inject methylene blue directly through the bronchial stump with a needle (10) while others, as ourselves, inject the dye via Electromagnetic Navigation Bronchoscopy (ENB). However, identification is less accurate (Figure 13) and ENB procedure takes time (mean time: 40 minutes).
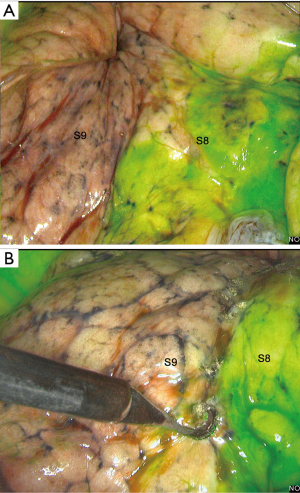
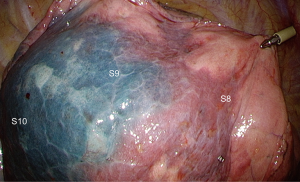
Although division of the intersegmental plane by cautery has been described, most authors use stapling to reduce the likelihood of air leakage and their inherent complications (11).
To prevent folding the parenchyma, stapling should be done thoroughly and step by step, starting from the diaphragmatic side and progressing in a cephalad direction (12). Stapling the ISP is tedious because, as described by Sato et al. stapling is not “linear” or “V-shaped” as for other segmentectomies but “3-dimensional” (13) because of the particular pyramidal shape of the basal segments. Therefore, if stapling is attempted with only a limited number of firing, it will fold the parenchyma. To prevent this, one should start from the diaphragmatic side, separating S8 from S9 and then S8 from S10, thus creating a “Mercedes-Benz mark” design (13). Once this step has been done, stapling is completed, starting from the converging point of the “Mercedes-Benz star” towards the fissure, following the cautery dots that have been applied during ICG marking (Figure 14). Figure 15 demonstrates the final aspect during reventilation.

Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alessandro Brunelli) for the series “Uncommon Segmentectomies” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Uncommon Segmentectomies” was commissioned by the editorial office without any funding or sponsorship. DG is consultant for an instrument manufacturer (Delacroix Chevalier). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gossot D, Lutz JA, Grigoroiu M, et al. Unplanned Procedures During Thoracoscopic Segmentectomies. Ann Thorac Surg 2017;104:1710-7. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Kawatani N, et al. Thoracoscopic lateral and posterior basal (S9 + 10) segmentectomy using intersegmental tunnelling. Eur J Cardiothorac Surg 2017;51:790-1. [PubMed]
- Nakao K, Sato M, Nitadori J, et al. Bilateral segmentectomies using virtual-assisted lung mapping (VAL-MAP) for metastatic lung tumors. Surg Case Rep 2017;3:104. [Crossref] [PubMed]
- Yang Q, Xie B, Hu M, et al. Thoracoscopic anatomic pulmonary segmentectomy: a 3-dimensional guided imaging system for lung operations. Interact Cardiovasc Thorac Surg 2016;23:183-9. [Crossref] [PubMed]
- Guigard S, Triponez F, Bédat B, et al. Usefulness of near-infrared angiography for identifying the intersegmental plane and vascular supply during video-assisted thoracoscopic segmentectomy. Interact Cardiovasc Thorac Surg 2017;25:703-9. [Crossref] [PubMed]
- Nomori H, Okada M. Illustrated anatomical Segmentectomy for Lung Cancer. Tokyo: Springer-Verlag, 2012.
- Gossot D. Atlas of endoscopic major pulmonary resections - 2nd edition. Springer-Verlag, 2018.
- Ramos R, Girard P, Masuet C, et al. Mediastinal lymph node dissection in early-stage non-small cell lung cancer: totally thoracoscopic vs thoracotomy. Eur J Cardiothorac Surg 2012;41:1342-8; discussion 1348. [Crossref] [PubMed]
- Gossot D, Seguin-Givelet A. Dissection of S9+10 arteries and exposure of the corresponding bronchi. Asvide 2018;5:717. Available online: http://www.asvide.com/article/view/26757
- Zhang Z, Liao Y, Ai B, et al. Methylene blue staining: a new technique for identifying intersegmental planes in anatomic segmentectomy. Ann Thorac Surg 2015;99:238-42. [Crossref] [PubMed]
- Ojanguren A, Gossot D, Seguin-Givelet A. Division of the intersegmental plane during thoracoscopic segmentectomy: is stapling an issue? J Thorac Dis 2016;8:2158-64. [Crossref] [PubMed]
- Yamanashi K, Okumura N, Otsuki Y, et al. Stapler-Based Thoracoscopic Basilar Segmentectomy. Ann Thorac Surg 2017;104:e399-402. [Crossref] [PubMed]
- Sato M, Murayama T, Nakajima J. Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP). J Thorac Dis 2016;8:S716-30. [Crossref] [PubMed]
- Gossot D, Seguin-Givelet A. Reinflation at completion of segmentectomy. Asvide 2018;5:718. Available online: http://www.asvide.com/article/view/26758
Cite this article as: Gossot D, Seguin-Givelet A. Thoracoscopic right S9+10 segmentectomy. J Vis Surg 2018;4:181.