
Microlobectomy: completely portal pulmonary lobectomy
Introduction
Endoscopic pulmonary lobectomy is 25 years old (1-4) and it is living its second period of enthusiasm, witnessed by an exponential development of techniques during the last few years (5-9). This is due to the evolution of the surgical expertise but also to the marketing and adoption of novel technologies. For example, the development of 5, or even 3 mm, diameter instruments and cameras allows to reduce the ports’ size and so the invasiveness of the surgery. This is particularly valuable when applied to thoracic surgery, where the access to the pleural cavity is limited by the intercostal spaces. There are several studies and randomized controlled trials which established that reducing the compression of the intercostal bundles during the procedure, or during the closure, reduce significantly the post-operative pain and so enhance the recovery (10-12). This is only partially applied during video-assisted thoracic surgery (VATS) pulmonary lobectomy, which by definition does not allow the use of a rib spreader in order to reduce the compression on the intercostal neurovascular bundle. Nevertheless, some degree of pressure is applied: by large caliper (10 or 12 mm diameter) ports and through the mini-thoracotomy utility incision, for delivery of the specimen. Furthermore, the intercostal chest drain is still reason of significant post-operative pain. The intercostal space, in fact, is only 8 to 10 mm wide and it is coursed by the intercostal nerve and its branches. At least 2 nerves are present along most of the space, one inferior to each rib and the collateral branch over the rib below. These nerves provide innervation to the superficial layers of the skin dermatomes, but also provide a rich innervation to the deeper layer, ensuring innervation to the parietal pleura. In the last few years we assisted to a rediscovered interest for the subxiphoid incision to gain access to the plural cavity (13). This tool is not new in thoracic surgery as it was initially described by Mineo et al. in the late nineties for bilateral pulmonary hand assisted metastasectomy (14,15). More recently major lung resections were performed through the subxiphoid port (16) and the Shanghai group described 153 anatomical resections through this access (17).
The above considerations gave inspiration to the technique conceived by Joel Dunning and described by Dunning et al. in 2017 (18). The hypothesis is: if we eliminate the utility incision, if we perform only 5 mm incisions in the intercostal spaces, if we do not place an intercostal drain and if we do not remove the large specimen through the small intercostal space, this will result in minimal trauma of the intercostal nerves and so in reduced post-operative pain. Also, we do not use diathermy blade on the periosteum of the rib and on the parietal pleura. All these maneuvers should result in a significant reduction of the post-operative pain, and, consequently, in a reduction of the post-operative complications related to the chest wall dynamic dysfunction, pulmonary atelectasis, impaired cough effort, secretions’ retention, adrenergic secretion with tachycardia or atrial fibrillation, prolonged air leak related to poor mobility and all the other complications related to poor mobility and prolonged hospital stay. The procedure was named ‘Microlobectomy’ referring to the small size of the intercostal incisions, in the attempt to reduce the surgical trauma and improve the patients’ outcome.
Methods
Data collection and analysis
This manuscript, aided by the video (Figure 1), is a descriptive, non-comparative, consecutive series of patients underwent major pulmonary resection, for different conditions, in a single centre, single surgeon experience (Joel Dunning) from January 2014 until December 2016. The data were prospectively collected using the bespoke software for medical records at James Cook University Hospital, Middlesbrough, UK. The patients included were treated with the adoption of a novel minimally invasive technique which was recently described in a multi-centric consecutive series of 72 patients (18) and is summarized again below.

Surgical technique
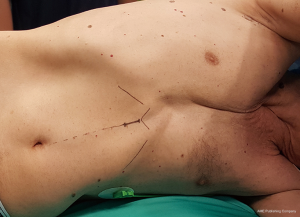
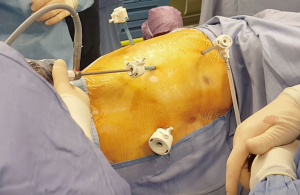
The general anesthesia is maintained with a selective lung ventilation. The patient, in a supine position, is marked over the midline from the xiphisternum to the umbilicus. The patient is then positioned on a lateral decubitus as per traditional lobectomy (Figure 2). The only difference is that the sterile drapes must leave the midline exposed. We always mark the patient prior to position as the superficial layers usually slide over the muscular layer so that the linea alba does not correspond to the skin midline in a lateral position. After injection of local anesthetic (Chirocaine) the first port is usually placed in the fourth intercostal space, in the same position where we would normally place the utility incision for an anterior approach VATS lobectomy, such as between the inferior angle of the scapula and the nipple in the 4th intercostal space. After a 5 mm skin incision, we position the camera inside the transparent plastic trocar (Kii-Fios first entry port, Applied Medical, Rancho Santa Margarita, CA, USA) and this is pushed, through the subcutaneous tissues and toward the pleural cavity, under direct vision. The CO2 insufflation is on, and set at a pressure of 5 mmHg and at 5 liter/minute flow rate. As soon as the tip of the trocar perforates the parietal pleura, the carbon dioxide pressure pushes the lung away and display eventual adhesions. Once the pleural cavity is entered, and the patient remains hemodynamically stable, the pressure could be increased up to 10 mmHg. The trocar can be used to release eventual adhesions close to the port site. The trocar is removed and the camera is then directed at the inferior border of the sternum. The subxiphoid port will now be performed. After a 12 mm skin incision on the pre-marked spot, the xiphisternum is palpated and the linea alba is incised inferior to it. We think that is very important to stay on the fibrous tissue of the linea alba and do not divert in the rectus abdominis as this would be reason for bleeding and pain. The index finger is pushed behind the xiphoid process and vertically up, behind the body of the sternum, and then towards the side of the surgery. At this stage the finger should appear under the pericardial adipose tissue, or the parietal pleura, and access to the chest cavity is easily gained pushing the finger through (Figure 1). A 12 mm trocar will follow. If the procedure is performed as described and as showed in the video, the trocar is always well above the diaphragm. Access to the pleural cavity is never gained through the diaphragm, but over it. Further 2 mm × 5 mm trocars are placed inferior and posterior to the first one, similarly to an anterior VATS approach (Figure 3) or according to the surgeon’s usual preferences. We always inject local on the sites prior to incise. The 4 trocars should maintain a sealed surgical field, which permits the use of the carbon dioxide throughout the procedure. Intercostal blocks are now performed under direct vision. Alternatively, an epipleural catheter could be placed. We reserved the catheter to high risk patients or fragile patients where we predicted impaired post-operative mobility. Otherwise, we favored intercostal blocks in order to reduce the patient’s postoperative devices. Since now on the procedure is similar to a normal VATS lobectomy with the only difference that all the instruments used in the intercostal spaces must be 5 mm in diameter or less. Some of the vessels, in this series, were stapled with the 5 mm MicroCutter 5/80 (Dextera Surgical, Inc., Redwood City, CA, USA). This device is no longer on the market and, as an alternative, clips, energy devices with 5 mm shaft or traditional tri-stapler through the subxiphoid port are now adopted. The subxiphoid port can be used for retraction, suction and, importantly, for large tools like the 12 mm tri-stapler. The least is essential for completion of the fissures and resection of the bronchus. The subxiphoid port, which is at the distal end of the oblique fissures, both on the left and on the right side, offers a suitable angle to tackle all the hilar structures and the fissures. At the end of the procedure the specimen, once placed into an endo-bag, is drained of blood and air and it is then removed from the enlarged subxiphoid port. The wound spreads in a circular fashion, and is not constricted by the ribs. The intercostal nerves are not traumatised. Also, a 24 Fr chest drain is placed through this incision, at its proximal end (Figure 4).
Post-operative care
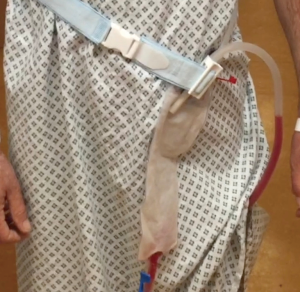
Enhanced recovery pathway is applied and in keeping with the attitude of minimally invasiveness of the surgery. Arterial line, if necessary at all, is usually removed in recovery room. When there is no significant air leakage, the chest drain can be connected to an ambulatory flutter bag, with Heimlich valve, directly in the operating room (Figure 5). This is removed on the following morning if good lung re-expansion is achieved. Routinely a patient-controlled analgesia (PCA) with morphine (1 mg every 5 minutes) is set up in the operating room but this should be discontinued in favor of oral analgesics 4 to 6 hours after surgery. The use of opioids should be limited and discontinued as soon as appropriate, possibly on the afternoon of the operation. The oral analgesia is usually with 1 gram of paracetamol, four times a day, combined with oxycodone 10 mg twice a day, the least can step down to Codeine four times a day. Some patients benefit from 5% lignocaine patches overnight close to the wounds site. The patient is encouraged to sit or even to walk on the same day. The physiotherapists visit her/him twice a day, performing breathing and coughing exercises and mobilization.
Results
Eighty-two patients underwent Microlobectomy at James Cook University Hospital from January 2014 and until December 2016. Mean age was 66 years (range, 27–82 years). Sixty-nine operations were performed for pulmonary malignancy (various stages, ranging from Ia to IIIb). Fifty-three patients underwent a right sided procedure and 29 a left sided, namely the procedures were: 30 right upper lobectomies, 9 right middle, 13 right lower, 15 left upper, 7 left lower, 4 trisegmentectomy, 2 lingulectomy, 1 right and 1 left pneumonectomy. Further patients’ clinical details are showed in Table 1. In 12 cases the procedures were associated to other procedures, like frozen section for diagnosis, in 5 cases, and wedge resections in a different lobe in 7 individuals. Six patients sustained previous thoracic surgery on the same side, and so they were a re-do VATS surgery.
Table 1
| Clinical details | Number of patients, n (%) |
|---|---|
| Dyspnoea score | |
| Grade 1 | 34 (41.5) |
| Grade 2 | 34 (41.5) |
| Grade 3 | 13 (15.9) |
| Grade 4 | 1 (1.2) |
| Performance status | |
| PS 0 | 22 (26.8) |
| PS 1 | 52 (63.4) |
| PS 2 | 8 (9.8) |
| ASA grade | |
| Grade 1 | 4 (4.9) |
| Grade 2 | 19 (23.2) |
| Grade 3 | 57 (69.5) |
| Grade 4 | 2 (2.4) |
| History of IHD | 14 (17.1) |
| Smoker at the time of surgery | 19 (23.2) |
| Ex-smoker | 52 (63.4) |
| Never smoked | 11 (13.4) |
| COPD | 39 (47.6) |
| Creatinine>110 | 6 (7.3) |
| Previous history of cancer | 24 (29.3) |
| Analgesia strategy | |
| Extra-pleural catheter | 52 (63.4) |
| PCA | 68 (82.9) |
IHD, ischemic heart disease; COPD, chronic obstructive pulmonary disease; PCA, patient-controlled analgesia.
In 75 patients the strategy of the 3 mm × 5 mm intercostals ports with the addition of the 12 mm subxiphoid port was adopted, as described above. In 1 case 4 mm × 5 mm ports were performed. In 2 cases 3 mm × 5 mm ports were associated to a subcostal port, rather than a subxiphoid one, finally in 4 cases the subxiphoid port was associated only to 2 mm × 5 mm intercostal ports. In 2 cases an Alexis port was placed in the subxiphoid assess. This was not a successful variation, as the Alexis does not allow the use of CO2, and the surgical field was narrowed. In 1 case a bariatric port was needed in the subxiphoid region. In 3 patients conversion to open surgery was required, which make up a conversion rate of 3.7%. Two cases were converted to anterolateral thoracotomy respectively for uncontrolled arterial bleeding and to seal a large air leak. The third case was a conversion to posterolateral thoracotomy for a controlled bleeding. Conversion to traditional, posterior approach, VATS was required in one case to sling the pulmonary artery. In 2 cases the posterior port was enlarged to 12 mm in order to use a larger stapling device through it. All patients had 1 chest drain.
The mean operative time was 189 minutes (range, 126–315 minutes). The blood loss was on average 136 mL (range, 7–1,000 mL). Thirty days mortality was 1.2%: 1 patient deceased for sepsis and renal failure in the intensive care unit. This patient was a high-risk patient, with history of aortic stenosis and emphysema, who developed chest sepsis 1 week after surgery. Surgical morbidities were pneumonia which required intravenous antibiotics in 12 patients (14.6%), atrial fibrillation in 3 cases (3.7%) and prolonged air leakage in 9 patients (11%, more than 5 days). Importantly, 17 patients (20.7%) were discharged on the day after the procedure. 31patients (37.8%) went home on post-operative day 2. The median length of stay was 3 days (range, 1–34 days). Sixty-three patients (76.8%) went home within the first week from the procedure. The final histology is showed in Table 2.
Table 2
| Definitive histology | Number of patients |
|---|---|
| Pulmonary cancer | 69 (26 IA, 19 IB, 9 IIA, 6 IIB, 8 IIIA, 1 IIIB) |
| Primary pulmonary adenocarcinoma | 46 (66.7%) |
| Squamous cell lung cancer | 15 (21.7%) |
| Adenosquamous | 3 (4.3%) |
| Carcinoid | 4 (5.8%) |
| Atypical carcinoid | 1 (1.4%) |
| Benign diseases | 7 (2 chondroid hamartomas, 1 lung abscess, 1 silicosis, 1 rheumatoid nodules, 1 vascular hypertensive changes, 1 scarring) |
| Colorectal adenocarcinoma metastasis | 6 |
Conclusions
We believe that Microlobectomy is a valid alternative to traditional VATS techniques. It reduces the injury to the chest wall when performing pulmonary lobectomy. The project started, in 2014 at James Cook University Hospital, from the anatomical consideration that the intercostal space is 8 or maximum 10 mm. That was the rationale for reducing the ports to 5 mm or less. The strength of Microlobectomy is that the procedure of resection is substantially similar to a normal VATS approach, and also each surgeon could move the intercostal ports according to their preferences or patients need (posterior, anterior, bi-portal approaches, etc.). Secondly, the 5 mm instruments in the intercostal space should cause no post-operative pain and the subxiphoid retrieval avoids to incise the intercostal muscles and to compress the neurovascular bundles. The diathermy is not used on the parietal pleura or ribs’ periosteum. Its limitation: relevant experience with VATS is needed before to start. We acknowledge that consolidated experience as VATS lobectomist is needed before to be confident with 5 mm instruments and abandon the utility port. Only one case in our series was performed by a resident. After these encouraging results we will continue to offer this approach and be able to report on a wider cohort of patients and have long term clinical and oncological outcomes.
Acknowledgments
The data of this manuscript were presented at the Society of Cardiothoracic Surgery of Great Britain and Ireland meeting, Glasgow 2018. The authors would like to thank all the staff involved in the care of the patients described, at James Cook University Hospital, Middlesbrough, UK.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.06.03). MM serves as an unpaid editorial board member of Journal of Visualized Surgery from Dec 2016 to Nov 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required by the institution, James Cook University Hospital. The procedure had standard indications and oncological principles and the patients were not involved in a formal trial.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Roviaro G, Varoli F, Vergani C, et al. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest 2004;126:725-32. [Crossref] [PubMed]
- Walker WS, Carnochan FM, Pugh GC. Thoracoscopic pulmonary lobectomy. Early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg 1993;106:1111-7. [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg 1993;56:1248-52; discussion 1252-3. [Crossref] [PubMed]
- Migliore M, Deodato G. A single-trocar technique for minimally-invasive surgery of the chest. Surg Endosc 2001;15:899-901. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. [Crossref] [PubMed]
- Zieliński M, Pankowski J, Hauer Ł, et al. The right upper lobe pulmonary resection performed through the transcervical approach. Eur J Cardiothorac Surg 2007;32:766-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Patel B, et al. Intercostal muscle flap reduces the pain of thoracotomy: a prospective randomized trial. J Thorac Cardiovasc Surg 2005;130:987-93. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Maniscalco LM. A nondivided intercostal muscle flap further reduces pain of thoracotomy: a prospective randomized trial. Ann Thorac Surg 2008;85:1901-6; discussion 1906-7.
- Cerfolio RJ, Price TN, Bryant AS, et al. Intracostal sutures decrease the pain of thoracotomy. Ann Thorac Surg 2003;76:407-11; discussion 411-2. [Crossref] [PubMed]
- Nardini M, Migliore M, Jayakumar S, et al. Subxiphoid port applied to robotic pulmonary lobectomies. J Vis Surg 2017;3:35. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Video-assisted approach for transxiphoid bilateral lung metastasectomy. Ann Thorac Surg 1999;67:1808-10. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Paci M, et al. Transxiphoid bilateral palpation in video-assisted thoracoscopic lung metastasectomy. Arch Surg 2001;136:783-8. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Dunning J, Elsaegh M, Nardini M, et al. Microlobectomy: A Novel Form of Endoscopic Lobectomy. Innovations (Phila) 2017;12:247-53. [Crossref] [PubMed]
- Nardini M, Bilancia R, Solli P, et al. A novel technique of video assisted lung surgery: Microlobectomy. Asvide 2018;5:635. Available online: http://www.asvide.com/article/view/26036
Cite this article as: Nardini M, Bilancia R, Solli P, Jayakumar S, Paul I, Migliore M, Dunning J. Microlobectomy: completely portal pulmonary lobectomy. J Vis Surg 2018;4:153.


