
Therapeutic strategies for jejunal diverticulitis: our experience and a review of the recent literature
Introduction
Excluding Meckel’s diverticulum, diverticulosis of the small bowel is an uncommon disease with variable clinical presentation. The prevalence increases with the age and ranges of incidence varies from 0.06% to 1.3% (1). The clinical presentations are various, such as chronic non-specific symptoms: intermittent abdominal pain, constipation, diarrhoea, dyspepsia and malnutrition; sometimes, in case of complications, it presents with an acute abdominal pain or gastrointestinal bleeding. Complications of jejunal diverticulitis (JD) are perforation (2.1% to 7%), that could lead to generalized peritonitis or localized peritonitis with a mesenteric abscess, acute intestinal obstruction (2.3–4.6%) and diverticular bleeding (2–8.1%) (2). Most times the correct diagnosis is reached when the disease becomes complicated, therefore a delayed diagnosis can be fatal. In fact, diverticular perforation is associated with a high mortality in up to 40% of patients (3). JD is usually diagnosed during emergency surgery; indeed, JD can be often missed at preoperative imaging exams (4,5). There is no consensus about therapeutic strategy and conservative management of the symptomatic jejunal diverticular disease. Here, due to the rarity we reported two cases of JD in elderly patients arrived to our emergency department due to an acute abdomen syndrome. We decided to review the recent literature due to a not standardized therapeutic approach.
Cases presentations
Case 1
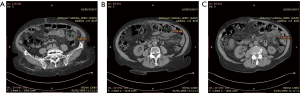
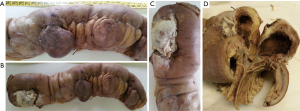
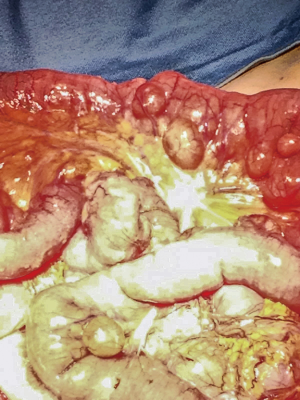
An 88-year-old woman arrived to our observation due to a diffused abdominal pain and fever (37.5 °C). The medical history of the patient reported arterial hypertension, insulin-dependent diabetes, chronic atrial fibrillation, colic diverticulosis and a previous surgical intervention of laparotomic cholecystectomy. Laboratory exams revealed only an increased white blood cell (WBC) count (WBC 13.300/mm) and C-reactive protein (CRP) (11.10 mg/dL). At physical examination, the abdomen presented a generalized tenderness with signs of peritonitis. A computed tomography (CT) demonstrated three round lesions containing faecal-like material mixed with gas (5.6 cm, 4.7 cm, 3.5 cm) within the mesentery, in the left side of abdomen (Figure 1). These lesions were seemed to communicate directly with adjacent loops of small bowel with a surrounding inflammatory reaction in peritoneum. Therefore, due to the acute clinical syndrome, we have decided to perform an exploratory laparotomy. We have found the presence of three diverticula, one of which was perforated. The patient was grade II in accordance to the modified Hinchey classification of jejunoileal diverticulitis proposed by Kaiser at al. (6). We performed a jejunal resection of about 50 cm with latero-lateral anastomosis by a linear stapler. Histopathological examination showed multiple diverticula on the jejunum with focal necrosis (Figure 2). Patient made uneventful recovery with discharge after 15 days from surgery.
Case 2
An 86-year-old woman with no significant medical history, that came to our Emergency Department with progressive worsening abdominal pain, associated with fever (37.6 °C), nausea and vomiting. Her physical examination revealed a diffuse abdominal pain with rebound tenderness. At laboratory exams an elevated WBC count (WBC 13.320/mm) and CRP (16.30 mg/dL) were observed. The CT scan showed a twisting of the mesenteric vessels and small bowel around the mesentery with mesenteric vessels creating the whirl sign suggestive of volvulus (whirlpool sign) with few enlarged mesenteric lymph nodes (Figure 3). Therefore, the patient was submitted to an emergency exploratory laparotomy, during which multiple diverticula were seen on the mesenteric side of jejunal segment at a distance of about 150 cm from the Treitz ligament (Figure 4). The patient was grade Ia in accordance to the modified Hinchey classification (6). Therefore, we have performed the excision of the involved jejunal segment containing diverticula and latero-lateral anastomosis. The histopathology exam revealed multiple diverticular pockets and spots of perivisceritis. The patient tolerated very well the surgical procedure and the post-operative period was uncomplicated.

Materials and methods
Since in literature there is not a univocal consensus on the treatment of JD, we have decided to perform a review of the recent literature to evaluate the best therapeutic strategy for this pathology. We have performed a PubMed research of the world literature between January 1st 2007, to December 31st 2017 (last 10 years), using the keyword “Jejunal Diverticulitis”. We have found 247 articles, of which about 100 were case reports. All papers in English and Italian reporting therapeutic strategy were included. We have excluded also articles concerning Meckel diverticula, colon and duodenum diverticula, the cases in which the treatment used were not reported, non-inflammatory complications of jejunal diverticular disease, and the multicenter descriptive studies in which individual patients could not be discerned. In case of multiple publications on the same group of patients, only the most complete paper was retained. All types of study design were included. There was no restriction on the patient number. The following data were analysed: year, sex, age (year), associated symptomatology, Hinchey modified classification, leukocytes (n° ×103/mL), CRP (mg/dL) and the treatment adopted. We have decided to classify every patient according to the modified Hinchey classification of jejunoileal diverticulitis proposed by Kaiser et al. (6). This classification has been used to distinguish different stages of acute diverticulitis based on clinical and operative findings. The stage Ia correspond to a confined peri-jejunoileal inflammation or phlegmon; the stage Ib correspond to a confined peri-jejunal abscess and a distant mesenteric abscess correspond to stage II; stage III was defined as generalized purulent peritonitis. The conservative treatment consists in an antibiotic coverage at broad spectrum, hydration with IV fluids and bowel rest.
Results
Based on our search criteria, we have identified 66 JD in literature reporting their treatment; with our two cases we have obtained a total of 68 cases (Table 1). Patients were 41 males and 27 females with a median age of 70.44 years and a standard deviation (SD) of ±15.19. In 32 patients the value of leukocytes was reported with a medium value of 16.51 (n° ×103/mL) and a SD ±4.67. In 17 patients was reported the value of CRP, of which the medium value is 16.19, and the SD ±9.02. We have reported the Hinchey’s stage in 61 patients, 33 of which had diverticulitis without evidence of abscess or perforation (stage Ia); 18 of these patients (54.5%) underwent open surgery with intestinal resection; only one of these underwent laparotomic exploration and wash out without resection; 11 (33.3%) patients received conservative treatment, that failed in 3 of these patients; 4 (12.1%) patients underwent laparoscopy surgery with intestinal resection, two of these were converted into laparotomy and one underwent laparoscopic exploration and wash out. A peri-jejunal abscess (stage Ib) was seen in 14 of 61 patients; 11 (78.6%) of which underwent laparotomic exploration, 9 of these with intestinal resection and in only one case a wash out was performed; 3 (21.4%) patient received non-operative treatment, one of which failed. Two patients had mesenteric abscess (stage II) and both received surgery treatment with intestinal resection, one by laparotomic approach and one by the laparoscopic one. Generalized peritonitis (stage III) was present in 12 patients, all of whom was treated surgically with intestinal resection, 11 of which by laparotomic approach and one by the laparoscopic one, which was then converted into laparotomy; one of these patients died due of a septic shock.
Table 1
| Reference | Year | Sex | Age (years) | Other symptoms in addition to abdominal pain | Leukocytosis (n° ×103/mL) | CRP (mg/dL) | Modified Hinchey classification grade | Treatment |
|---|---|---|---|---|---|---|---|---|
| Kubota T (8) | 2007 | F | 79 | Fever | NM | NM | III | Open surgery with resection |
| Grace PP (9) | 2007 | F | 66 | Vomit | NM | NM | Ia | Open surgery with resection |
| Buis CI (10) | 2008 | F | 50 | Fever | 11.4 | 14 | Ia | Open surgery with resection |
| Mortimer A (11) | 2008 | F | 90 | Rectal bleeding | Elevated | Elevated | Ib | Conservative |
| Balducci G (12) | 2008 | F | 49 | Vomit | NM | NM | Ia | Open surgery with resection |
| Leodolter A (13) | 2008 | M | 64 | Fever | Elevated | Elevated | Ia | Conservative |
| Staszewicz W (1) | 2008 | M | 88 | NM | 19 | 26 | Ia | Open surgery with resection |
| Veen M (14) | 2009 | M | 69 | NM | 14.3 | 24 | III | Open surgery with resection |
| Veen M (14) | 2009 | F | 67 | NM | Elevated | 4.6 | Ia | Conservative failed, then open surgery with resection |
| Bani Hani MN (15) | 2009 | M | 80 | NM | Normal | NM | Ia | Open surgery with resection |
| Wild JR (16) | 2009 | M | 75 | NM | NM | NM | Ia | Conservative |
| Graña L (17) | 2009 | M | 82 | Vomit | 18.2 | NM | Ib | Open surgery with resection |
| Graña L (17) | 2009 | F | 48 | Fever | 15.6 | NM | Ib | Open surgery with resection |
| Graña L (17) | 2009 | M | 75 | NM | Normal | NM | Ib | Open surgery with resection |
| Graña L (17) | 2009 | F | 83 | Vomit | NM | NM | Ib | Open surgery with resection |
| Graña L (17) | 2009 | F | 86 | NM | Normal | NM | Ib | Conservative failed, then open surgery with resection |
| Nejmeddine A (18) | 2009 | F | 47 | Vomit | 23.5 | NM | Ia | Open surgery with resection |
| Singh O (19) | 2009 | M | 74 | Fever and vomit | 19.3 | NM | Ia | Conservative |
| Singh O (19) | 2009 | F | 65 | Vomit | 17 | NM | Ia | Laparoscopic exploration, then open surgery with resection |
| Garg N (20) | 2009 | M | 57 | Constipation | NM | NM | Ia | Laparoscopic resection |
| Chugay P (21) | 2010 | M | 79 | Vomit | NM | NM | III | Open surgery with resection |
| Chugay P (21) | 2010 | F | 89 | NM | NM | NM | Ia | Open surgery with resection |
| Vanrykel F (22) | 2010 | F | 79 | Fever and nausea | 15 | 14.4 | III | Laparoscopic exploration and mini-laparotomy with resection |
| França M (23) | 2010 | M | 75 | NM | Elevated | Elevated | Ib | Open surgery with resection |
| Sakpal SV (24) | 2010 | F | 25 | Fever and vomit | 13.6 | NM | Ib | Open surgery with resection |
| Falidas E (25) | 2011 | M | 55 | Fever and vomit | 13 | 4.57 | Ia | Open surgery with resection |
| Nonose R (26) | 2011 | F | 86 | Vomit and constipation | 13 | NM | III | Open surgery with resection |
| Tan KK (27) | 2011 | M | 75 | Fever | NM | NM | III | Open surgery with resection |
| Tan KK (27) | 2011 | M | 84 | Vomit | NM | NM | III | Open surgery with resection |
| Tan KK (27) | 2011 | M | 70 | Fever | NM | NM | III | Open surgery with resection |
| Tan KK (27) | 2011 | M | 84 | Rectal bleeding | NM | NM | NM | Open surgery with resection |
| Tan KK (27) | 2011 | M | 72 | Rectal bleeding | NM | NM | NM | Open surgery with resection |
| Tan KK (27) | 2011 | M | 88 | Rectal bleeding | NM | NM | NM | Open surgery with resection |
| Garnet DJ (28) | 2011 | M | 80 | Feculent emesis | 27 | NM | Ia | Laparoscopic exploration, then open surgery with resection |
| Saberski E (29) | 2012 | M | 85 | Vomit and diarrhea | Normal | NM | Ia | Laparoscopic exploration, wash out and Conservative |
| Ferrarese A (30) | 2012 | F | 92 | NM | Elevated | 34.7 | III | Open surgery with resection |
| Ferreira-Aparicio FE (31) | 2012 | F | 65 | Fever | NM | NM | II | Open surgery with resection and terminal ileostomy |
| Singal R (32) | 2012 | M | 63 | Vomit and constipation | 18 | NM | Ib | Open surgery and wash out |
| Aydin I (33) | 2013 | F | 74 | Nausea and vomit | 13.2 | NM | Ia | Open surgery with resection |
| Zamani A (34) | 2013 | F | 63 | Fever | 11.1 | 13.9 | Ia | Conservative; then clinic worsening, abscess (Ib) and resection |
| Terada T (35) | 2013 | F | 67 | Melena | Elevated | 11.21 | NM | Open surgery with resection |
| Ojili V (36) | 2013 | M | 75 | Nausea; diarrhea | 21 | 30 | Ib | Open surgery with resection |
| Corcelles R (37) | 2014 | F | 63 | Fever; | 15 | 19 | II | Laparoscopic resection |
| Fresow R (38) | 2014 | M | 73 | Nausea and vomit | 32 | NM | Ia | Open surgery, drainage and conservative |
| Xu XQ (39) | 2014 | M | 86 | Hematochezia | NM | NM | Ia | Open surgery with resection |
| Levack MM (40) | 2014 | M | 77 | NM | 11 | NM | Ib | Conservative |
| Fidan N (41) | 2015 | M | 67 | Fever | NM | NM | Ia | Conservative |
| Kassir R (42) | 2015 | M | 79 | Fever | 16 | NM | III | Open surgery with resection |
| Natarajan K (43) | 2015 | M | 56 | Fever; vomit | NM | NM | III | Open surgery with resection |
| Khan HS (44) | 2015 | M | 33 | Fever; vomit | NM | NM | Ia | Open surgery with resection |
| De Minicis S (45) | 2015 | M | 60 | Melena | Severe anemia | NM | NM | Conservative |
| Blake-Siemsen JC (46) | 2016 | M | 53 | Melena | Severe anemia (Hb: 4.36 mg/dL) | NM | NM | Open surgery with resection |
| Harbi H (47) | 2016 | M | 31 | Severe hypothermia and septic shock | Severe leukopenia | NM | III | Open surgery with resection |
| Nakatani K (48) | 2016 | M | 37 | Fever | 20.7 | 2.6 | Ia | Conservative |
| Ghrissi R (49) | 2016 | M | 72 | Vomit | NM | NM | Ia | Open surgery with resection |
| Tenreiro N (50) | 2016 | M | 81 | Fever | 13.9 | 10 | Ib | Open surgery with resection |
| Aydin E (51) | 2016 | M | 69 | NM | 15 | 17.9 | Ia | Open surgery with resection |
| Walter BM (52) | 2016 | F | 83 | NM | 12 | 22 | Ia | Open surgery with resection |
| Walter BM (52) | 2016 | M | 56 | NM | 15.2 | NM | Ib | Open surgery with resection |
| Mohi RS (53) | 2016 | M | 62 | Constipation; vomit | 18.6 | NM | Ia | Open surgery with resection |
| Kumar D (54) | 2017 | M | 68 | Constipation | Elevated | NM | Ia | Open surgery with resection |
| Kumar D (54) | 2017 | F | 60 | Melena | Anemia (Hb: 7.2 mg/dL) | NM | NM | Open surgery with resection |
| Grubbs J (55) | 2017 | M | 90 | Fever; nausea and vomit; diarrhea | Normal | NM | Ia | Conservative failed, then open surgery with resection |
| Ejaz S (56) | 2017 | M | 87 | Fever | 13 | NM | Ia | Conservative |
| Ejaz S (56) | 2017 | F | 78 | Diarrhea | 16.4 | NM | Ia | Conservative |
| Ejaz S (56) | 2017 | F | 76 | Constipation and vomit | 19.9 | NM | Ia | Conservative |
| Our case report | 2018 | F | 88 | Fever | 13.3 | 10.1 | II | Open surgery with resection |
| Our case report | 2018 | F | 86 | Fever; nausea and vomit | 13.3 | 16.3 | Ia | Open surgery with resection |
NM, not mentioned; F, female; M, male; CRP, C-reactive protein.
Discussion
The first description in literature of a jejunal diverticulum is due to von Soemmerring (57) in 1974. Nowadays this disease is considered as a “pseudodiverticula”, characterized by herniation of the mucosa and the submucosa through the muscular layer of the bowel wall. Diverticula develop in correspondence of the blood vessels penetration sites, considered as the weak points of the mesenteric border of the small bowel. Until today, the etiology is not clear with different hypotheses about its origin. Three different types of microscopic abnormalities have been hypothesized by diver authors: visceral neuropathy, visceral myopathy and progressive systemic sclerosis. In fact, Kassir et al. (42) focused on abnormalities in the smooth muscles or myenteric plexus, on intestinal dyskinesis and on high intraluminal pressures. Weston et al. (58) reported an important incidence of small bowel diverticula (42%) in patients with progressive systemic sclerosis. The prevalence of small bowel diverticula ranges from 0.06% to 1.3% with a peak incidence at the sixth and seventh decades with a male predominance (4). This pathology is more common in proximal jejunum because of the larger size of the vasa recta in this area. In the study of Liu et al. based on 28 patients, 55% of the diverticula were found in the jejunum, 38% in the ileum and 7% in both (59). This distribution of diverticula is similar to the findings of others author. The size of the diverticula ranges from a few millimeters to more than 3 cm (25); in the literature there is a case of a jejunal diverticulum measuring about 26 cm (60). The disease often presents with non-specific symptoms like intermittent abdominal pain, dyspepsia, bloating or abdominal fullness, constipation, diarrhoea, malnutrition, anemia due to iron deficiency or megaloblastic anemia. Edwards in 1949 described a symptom triad observed as “flatulent dyspepsia” characterized by epigastric pain, abdominal discomfort and flatulence 1 or 2 hours after meals (61). Complications of JD include perforation, with an incidence of 2.1–7%, acute intestinal obstruction (2.3–4.6%) and diverticular bleeding (2–8.1%) (2). Other types of complications are also possible such as abdominal abscesses, fistulas and hepatic abscesses (62). We should keep in mind that diverticulitis is not always the cause of a perforation. In some cases, perforation can be caused by penetration of the intestinal wall by a foreign body or by a blunt trauma to the abdominal wall (63). Nonose at al. reported a case about a small bowel perforation caused by an enterolith 12 cm in length, localized inside one of jejunal diverticula (26).
After the onset of complications, diverticulitis manifests itself with a picture of an acute abdomen that mimics other pathologies such as colonic diverticulitis, appendicitis or acute cholecystitis. The differential diagnosis may include various diseases related to different etiologies: cancer (with or without perforation), foreign body perforation, traumatic hematoma, nonsteroidal anti-inflammatory drug (NSAID) abuse ulceration, and Crohn’s disease. Suspicion of jejunal diverticulosis is difficult and often the diagnosis is missed or delayed, thus increasing the mortality rate that in the past was 24% (64). Nowadays the mortality has been minimized because of the improvement of the diagnostic, pharmaceutical and surgical protocols, but preoperative diagnosis of JD is still rarely made. The jejunum is difficult to examine with the endoscopic methods, although we can exclude other causes of obstruction or bleeding. Therefore, the diagnosis of these diverticula is often only radiographic (26). Ultrasound is usually hindered by the presence of meteorism or adiposity; hence, this method alone is not suitable for JD diagnosis (65). Plain radiographic findings are non-specific in the diagnosis of JD, although Nobles et al. described a characteristic triad of clinical and radiographic findings: abdominal pain, anemia and intestinal segmental dilatation in the epigastrium or in the left upper abdomen (66). Only in cases of complicated JD, such in case of perforation, abdominal X-ray series demonstrate distension of small bowel, air-fluid and pneumoperitoneum. Multi-slice CT seems to be promising in diagnosing jejunal diverticula. In fact, it shows a focal area containing extraluminal air bubbles or air fluid levels in contiguity with an adjacent dilated small bowel loop, with thickening of the intestinal wall or an inflammatory process or an abscess adjacent to a jejunal loop with oedema of the surrounding mesenteric fat (17). CT is the most useful tool for confirming the diagnosis and should ideally be performed with oral and rectal contrast to differentiate the origin of diverticula from large or small bowel. Furthermore, thanks to the distensions and the opacification of the bowel by the administration of positive oral contrast, it is easier to assess the thickening of the bowel wall and to differentiate between intra- and extra-luminal structures. Magnetic resonance enterography (MRE) is quite useful for the diagnosis of JD, especially when CT with oral contrast is not contributory, but it remains exceptional to use this method in emergency cases.
There is no consensus on therapeutic strategy and conservative management of the symptomatic jejunal diverticular disease. Management depends on patients’ symptoms. If the inflammation is mild, the medical management may be attempted with bowel rest and antibiotics. In fact, Levack et al. reported a case with a successful non-operative management of a perforated jejunoileal diverticulum, which presented with localized abdominal symptoms and signs (40). In this case, the applied non-operative treatment consisted in a broad-spectrum antibiotic coverage utilizing IV ampicillin, ciprofloxacin and metronidazole, hydration with IV fluids and bowel rest. In case of intraperitoneal collections, other supportive measures may be suitable such as a CT-guided aspiration and drainage (67). Surgery with primary anastomosis is mandatory for intestinal resection, taking away the perforated diverticulum, in two situations: failure or unfeasibility of percutaneous drainage and in case of generalized peritonitis (68).
Both our cases presented with pictures of acute abdomen, one of which probably due to an intestinal volvulus (Ia) and the other with generalized peritonitis due to three mesenteric abscess (II). For these reasons, as the general clinical conditions of the patients were mediocre but still able to withstand a surgery, we have decided to execute an explorative laparotomy with intestinal resection and primary anastomosis. If diverticula extend over a long section of intestine or they are multiple, resection may have to be limited to include only the segment containing the perforated diverticulum and to leave a segment of small bowel that still contains non-perforated diverticula to avoid short-bowel syndrome. Furthermore, we should keep in mind that diverticula may recur in a patient undergone a segmental intestinal resection since the mechanism of diverticula formation still remains. Other surgical techniques have been performed in the past for JD such as suturing the perforation (with omental patch closure) and invaginating the diverticulum with a suture; these techniques have been abandoned since they present high mortality rates (69). Nowadays, laparoscopy becomes a valid diagnostic approach for complicated cases and this tool affords a variety of minimally invasive and conservative treatment options. Saberski et al. (29) reported a case of no perforated JD treated with laparoscopic lavage, postoperative bowel rest and intravenous antibiotics. Diagnostic laparoscopy is very useful in evaluating patients with a complicated course. This surgical technique is quickly convertible in laparotomy and it can function as a guide pointing out the area of the intestinal complication, avoiding this way larger abdominal incision and minimizing the time of the operation. A total laparoscopic treatment of a large (8 cm) jejunal diverticulum has been recently reported (20), with a laparoscopic resection of jejunal diverticulum and an intracorporeal side-to-side.
From the data of our review the patients in the stage Ia 66.7% of the surgeons preferred the surgical treatment, while 33.3% preferred the conservative one; 3 of these conservative approaches failed; the laparoscopic approach was used by 12.2% of surgeons, half of whom had to convert into laparotomy. For patients presenting with a mesenteric localized abscess, 78.57% of surgeons preferred the open surgery with intestinal resection, while the remaining 21.43% opted for non-operative approach, with only one case of failure. In cases of mesenteric abscess and generalized peritonitis, all surgeons decided to operate the patients and perform an intestinal resection.
Conclusions
Jejunoileal diverticulitis is frequently overlooked as a possible source of abdominal infection in the elderly patient and, because of its relative rarity; complications that come with it create technical dilemmas for the surgeon. There are different therapeutic approaches depending on the severity of the disease and the general clinical condition of the patient. Non-surgical treatment is usually sufficient for JD without abscess or peritonitis (stage Ia), although there is the probity that the treatment will fail. Emergency surgical treatment with resection of affected intestinal segment with primary anastomosis is mandatory in case of complications as perforation, abscesses and obstruction (stage Ib, II and III). From our review, we can deduce that most surgeons preferred the open approach to the laparoscopic one. Although laparoscopic techniques are improving in recent times, the exploratory laparotomy is still the best approach to an acute abdomen.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.07.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors declare that all procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013. Informed consent was obtained from the patient for being included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Staszewicz W, Christodoulou M, Proietti S, et al. Acute ulcerative jejunal diverticulitis: case report of an uncommon entity. World J Gastroenterol 2008;14:6265-7. [Crossref] [PubMed]
- Harbi H, Kardoun N, Fendri S, et al. Jejunal diverticulitis. Review and treatment algorithm. Presse Med 2017;46:1139-43. [Crossref] [PubMed]
- Peters R, Grust A, Gerharz CD, et al. Perforated jejunal diverticulitis as a rare cause of acute abdomen. Eur Radiol 1999;9:1426-8. [Crossref] [PubMed]
- Lempinen M, Salmela K, Kemppainen E. Jejunal diverticulosis: a potentially dangerous entity. Scand J Gastroenterol 2004;39:905-9. [Crossref] [PubMed]
- Makris K, Tsiotos GG, Stafyla V, et al. Small intestinal nonmeckelian diverticulosis. J Clin Gastroenterol 2009;43:201-7. [Crossref] [PubMed]
- Kaiser AM, Jiang JK, Lake JP, et al. The management of complicated diverticulitis and the role of computed tomography. Am J Gastroenterol 2005;100:910-7. [Crossref] [PubMed]
- Fleres F, Viscosi F, Bertilone E, et al. The wirple sign in CT scan. Asvide 2018;5:633. Available online: http://www.asvide.com/article/view/26030
- Kubota T. Perforated jejunal diverticulitis. Am J Surg 2007;193:486-7. [Crossref] [PubMed]
- Crace PP, Grisham A, Kerlakian G. Jejunal diverticular disease with unborn enterolith presenting as a small bowel obstruction: a case report. Am Surg 2007;73:703-5. [PubMed]
- Buis CI, Hofker HS, Nieuwenhuijs VB. Diverticulitis of the jejunum, an uncommon diagnosis. Dig Surg 2008;25:83-4. [Crossref] [PubMed]
- Mortimer A, Harding J, Roach H, et al. Jejunal diverticulitis: an unusual cause of an intra-abdominal abscess - coronal Computed Tomography reconstruction can aid the diagnosis. J Radiol Case Rep 2008;2:15-8. [Crossref] [PubMed]
- Balducci G, Dente M, Cosenza G, et al. Multiple giant diverticula of the foregut causing upper gastrointestinal obstruction. World J Gastroenterol 2008;14:3259-61. [Crossref] [PubMed]
- Leodolter A, Zielinski D, Borkenstein D, et al. Diagnosis of jejunal diverticulitis by oral single-balloon enteroscopy. Am J Gastroenterol 2008;103:2405-6. [Crossref] [PubMed]
- Veen M, Hornstra BJ, Clemens CH, et al. Small bowel diverticulitis as a cause of acute abdomen. Eur J Gastroenterol Hepatol 2009;21:123-5. [Crossref] [PubMed]
- Bani Hani MN, AlWaqfi NR, Heis HA, et al. Jejunal disorders: potentially lethal causes of acute abdomen are still overlooked. Surg Laparosc Endosc Percutan Tech 2009;19:39-42. [Crossref] [PubMed]
- Wild JR, Shiwani MH, Ullah Q. Jejunal diverticulitis. J Coll Physicians Surg Pak 2009;19:120-2. [PubMed]
- Graña L, Pedraja I, Mendez R, et al. Jejuno-ileal diverticulitis with localized perforation: CT and US findings. Eur J Radiol 2009;71:318-23. [Crossref] [PubMed]
- Nejmeddine A, Bassem A, Mohamed H, et al. Complicated jejunal diverticulosis: A case report with literature review. N Am J Med Sci 2009;1:196-9. [PubMed]
- Singh O, Gupta SS, Shukla S, et al. Jejunal diverticulae: reports of two cases with review of literature. Indian J Surg 2009;71:238-44. [Crossref] [PubMed]
- Garg N, Khullar R, Sharma A, et al. Total laparoscopic management of large complicated jejunal diverticulum. J Minim Access Surg 2009;5:115-7. [Crossref] [PubMed]
- Chugay P, Choi J, Dong XD. Jejunal diverticular disease complicated by enteroliths: Report of two different presentations. World J Gastrointest Surg 2010;2:26-9. [Crossref] [PubMed]
- Vanrykel F, Pattyn P, Vuylsteke P, et al. Perforated jejunal diverticulitis: a rare presentation of acute abdomen. Acta Chir Belg 2010;110:367-9. [Crossref] [PubMed]
- França M, Certo M, Silva D, et al. Elderly patient with acute, left lower abdominal pain: perforated jejunal diverticulitis (2010:7b). Eur Radiol 2010;20:2541-5. [Crossref] [PubMed]
- Sakpal SV, Fried K, Chamberlain RS. Jejunal Diverticulitis: A Rare Case of Severe Peritonitis. Case Rep Gastroenterol 2010;4:492-7. [Crossref] [PubMed]
- Falidas E, Vlachos K, Mathioulakis S, et al. Multiple giant diverticula of the jejunum causing intestinal obstruction: report of a case and review of the literature. World J Emerg Surg 2011;6:8. [Crossref] [PubMed]
- Nonose R, Valenciano JS, de Souza Lima JS, et al. Jejunal Diverticular Perforation due to Enterolith. Case Rep Gastroenterol 2011;5:445-51. [Crossref] [PubMed]
- Tan KK, Liu JZ, Ho CK. Emergency surgery for jejunal diverticulosis: our experience and review of literature. ANZ J Surg 2011;81:358-61. [Crossref] [PubMed]
- Garnet DJ, Scalcione LR, Barkan A, et al. Enterolith ileus: liberated large jejunal diverticulum enterolith causing small bowel obstruction in the setting of jejunal diverticulitis. Br J Radiol 2011;84:e154-7. [Crossref] [PubMed]
- Saberski E, Novitsky YW. Laparoscopic diagnosis and management of an acute jejunal diverticulitis. Surg Laparosc Endosc Percutan Tech 2012;22:e18-20. [Crossref] [PubMed]
- Ferrarese A, Assoua C, Ahmad K, et al. Perforated jejunal diverticulitis. Clin Res Hepatol Gastroenterol 2012;36:99-100. [Crossref] [PubMed]
- Ferreira-Aparicio FE, Gutiérrez-Vega R, Gálvez-Molina Y, et al. Diverticular disease of the small bowel. Case Rep Gastroenterol 2012;6:668-76. [Crossref] [PubMed]
- Singal R, Gupta S, Airon A. Giant and multiple jejunal diverticula presenting as peritonitis a significant challenging disorder. J Med Life 2012;5:308-10. [PubMed]
- Aydin I, Pergel A, Yucel AF, et al. A rare cause of acute abdomen: jejuna diverticulosis with perforation. J Clin Imaging Sci 2013;3:31. [Crossref] [PubMed]
- Zamani A, Peters CJ, Chadwick SJ. The use of an ex vivo contrast study at the time of surgery to confirm the site of a perforated jejunal diverticulum. BMJ Case Rep 2013;2013: [Crossref] [PubMed]
- Terada T. Diverticulitis of multiple diverticulosis of the terminal ileum. Int J Clin Exp Pathol 2013;6:521-3. [PubMed]
- Ojili V, Parizi M, Gunabushanam G. Timely diagnosis of perforated jejuna diverticulitis by computed tomography. J Emerg Med 2013;44:614-6. [Crossref] [PubMed]
- Corcelles R, Pavel M, Lacy A. Perforated small bowel diverticulitis after gastric bypass. JSLS 2014;18:142-5. [Crossref] [PubMed]
- Fresow R, Vieweg H, Kamusella P, et al. Jejunal diverticulitis ascending to the duodenum as a rare cause of acute abdomen. J Clin Diagn Res 2014;8:RD07-8. [PubMed]
- Xu XQ, Hong T, Li BL, et al. Active gastrointestinal diverticulum bleeding diagnosed by computed tomography angiography. World J Gastroenterol 2014;20:13620-4. [Crossref] [PubMed]
- Levack MM, Madariaga ML, Kaafarani HM. Non-operative successful management of a perforated small bowel diverticulum. World J Gastroenterol 2014;20:18477-9. [Crossref] [PubMed]
- Fidan N, Mermi EU, Acay MB, et al. Jejunal Diverticulosis Presented with Acute Abdomen and Diverticulitis Complication: A Case Report Pol J Radiol 2015;80:532-5. [Crossref] [PubMed]
- Kassir R, Boueil-Bourlier A, Baccot S, et al. Jejuno-ileal diverticulitis: Etiopathogenicity, diagnosis and management. Int J Surg Case Rep 2015;10:151-3. [Crossref] [PubMed]
- Natarajan K, Phansalkar M, Varghese RG, et al. Jejunal diverticulosis with perforation - a challenging differential diagnosis of acute abdomen: case report. J Clin Diagn Res 2015;9:ED03-4. [PubMed]
- Khan HS, Ayyaz M. Jejunal diverticulosis presenting as an acute emergency. J Coll Physicians Surg Pak 2015;25:S20-1. [PubMed]
- De Minicis S, Antonini F, Belfiori V, et al. Small bowel diverticulitis with severe anemia and abdominal pain. World J Clin Cases 2015;3:462-5. [Crossref] [PubMed]
- Blake-Siemsen JC, Kortright-Farías M, Casale-Menier DR, et al. Digestive bleeding due to jejunal diverticula: A case report and literature review. Cir Cir 2017;85:34-9. [Crossref] [PubMed]
- Harbi H, Fendri S, Sahnoun M, et al. Fatal acute peritonitis due to perforated jejunal diverticulum. Presse Med 2017;46:246-7. [Crossref] [PubMed]
- Nakatani K, Kato T, Okada S, et al. Flare-Up Diverticulitis in the Terminal Ileum in Short Interval after Conservative Therapy: Report of a Case. Case Rep Surg 2016;2016:8162797 [Crossref] [PubMed]
- Ghrissi R, Harbi H, Elghali MA, et al. Jejunal diverticulosis: a rare case of intestinal obstruction. J Surg Case Rep 2016;2016: [Crossref] [PubMed]
- Tenreiro N, Moreira H, Silva S, et al. Jejunoileal diverticulosis, a rare cause of ileal perforation - Case report. Ann Med Surg (Lond) 2016;6:56-9. [Crossref] [PubMed]
- Aydın E, Yerli H, Avcı T, et al. One of the Rare Causes of Acute Abdomen Leading to Subileus: Jejunal Diverticulitis. Balkan Med J 2016;33:354-6. [Crossref] [PubMed]
- Walter BM, Winker J, Wagner M, et al. Complicated jejunal diverticulosis - a rare but important diagnosis to consider in abdominal pain: a report of three cases. Z Gastroenterol 2016;54:562-5. [PubMed]
- Mohi RS. Complicated Jejunal Diverticulosis: Small Bowel Volvulus with Obstruction. Iran J Med Sci 2016;41:548-51. [PubMed]
- Kumar D. Meenakshi. Complicated jejunal diverticulitis with unusual presentation. Radiol Case Rep 2017;13:58-64. [Crossref] [PubMed]
- Grubbs J, Huerta S. Perforated jejunal diverticulitis in a nonagenarian veteran: A case report. Int J Surg Case Rep 2017;40:77-9. [Crossref] [PubMed]
- Ejaz S, Vikram R, Stroehlein JR. Non-Meckel Small Intestine Diverticulitis. Case Rep Gastroenterol 2017;11:462-72. [Crossref] [PubMed]
- Baille M, von Soemmerring ST. Anatomie des krankhaften Baues: von einigen der wichtigsten Teile im menschlichen Körper. Berline: In Vossiche Buchhandlung, 1974.
- Weston S, Thumshirn M, Wiste J, et al. Clinical and upper gastrointestinal motility features in systemic sclerosis and related disorders. Am J Gastroenterol 1998;93:1085-9. [Crossref] [PubMed]
- Liu CY, Chang WH, Lin SC, et al. Analysis of clinical manifestations of symptomatic acquired jejunoileal diverticular disease. World J Gastroenterol 2005;11:5557-60. [Crossref] [PubMed]
- Takehito E, Tsuyoshi K, Ichiro H. A case of large diverticulum of the distal jejunum. J Jap Surg Assoc 2004;65:1850-4. [Crossref]
- Edwards HC. Diverticula of the small intestine. Br J Radiol 1949;22:437-42. [Crossref] [PubMed]
- Horesh N, Klang E, Gravetz A, et al. Jejunal Diverticulitis. J Laparoendosc Adv Surg Tech A 2016;26:596-9. [Crossref] [PubMed]
- Prakash C, Clouse RE. Acute ileal diverticulitis. Am J Gastroenterol 1998;93:452-4. [Crossref] [PubMed]
- de Bree E, Grammatikakis J, Christodoulakis M, et al. The clinical significance of acquired jejunoileal diverticula. Am J Gastroenterol 1998;93:2523-8. [Crossref] [PubMed]
- Schloericke E, Zimmermann MS, Hoffmann M, et al. Complicated jejunal diverticulitis: a challenging diagnosis and difficult therapy. Saudi J Gastroenterol 2012;18:122-8. [Crossref] [PubMed]
- Nobles ER Jr. Jejunal diverticula. Arch Surg 1971;102:172-4. [Crossref] [PubMed]
- Novak JS, Tobias J, Barkin JS. Nonsurgical management of acute jejunal diverticulitis: a review. Am J Gastroenterol 1997;92:1929-31. [PubMed]
- Kassahun WT, Fangmann J, Harms J, et al. Complicated small-bowel diverticulosis: a case report and review of the literature. World J Gastroenterol 2007;13:2240-2. [Crossref] [PubMed]
- Englund R, Jensen M. Acquired diverticulosis of the small intestine: case reports and literature review. Aust N Z J Surg 1986;56:51-4. [Crossref] [PubMed]
Cite this article as: Fleres F, Viscosi F, Bertilone E, Mazzeo C, Cucinotta E. Therapeutic strategies for jejunal diverticulitis: our experience and a review of the recent literature. J Vis Surg 2018;4:152.


