Current anesthesiological approach to mediastinal surgery
Introduction
The mediastinum constitutes probably the most sacred region of the whole human body because it contains most of the vital organs, including the heart, great vessels and pulmonary hilums.
There are many surgical procedures involving the mediastinum, some of them for diagnostic purpose but many other therapeutic procedures for different benign and malignant conditions, and usually are named depending on their location into anterior, middle or posterior mediastinum (Table 1), being the anterior masses the most life-threatening. Perioperative complications of these procedures are not negligible (14.3% in a retrospective cohort from 1994 to 2000) (1). Mortality rate for mediastinal procedures is very low (2) but should be kept in mind for huge anterior masses where it has been described (3-6).
Table 1
| Location | Benign | Malignant |
|---|---|---|
| Anterior | Thyroid | Thymic carcinoma |
| Cystic hygroma | Thyroid carcinoma | |
| Thymic cist | Seminoma | |
| Thymic hyperplasia | Mixed germ cell tumor | |
| Lymphoma | ||
| Thymoma | ||
| Middle | Cysts | Lymphoma |
| Benign adenopathy | Metastasis | |
| Hiatus hernia | Esophageal cancer | |
| Esophageal mass | Thyroid carcinoma | |
| Cardiac/vascular structure | ||
| Posterior | Schwannoma | Neuroblastoma |
| Neurofibroma | ||
| Chemodectoma | ||
| Foramen Bochdalek hernia |
Due to the heterogeneity of the underlying pathology and the potential compression of the central airway, great vessels and the heart, the anesthesiologist should keep in mind some preoperative considerations and some concerns about management of respiratory and cardiovascular parameters (7). There are also some special concerns for patients with miastenia gravis that should be kept in consideration in order to avoid postoperative complications and myasthenic crisis, and will be explained later.
Although initially considered mandatory to perform these procedures under orotracheal intubation and mechanical ventilation, some pathologies in selected patients can be performed under spontaneous ventilation in a non-intubated approach.
The aim of this review is to discuss main specific aspects of anesthesia for mediastinal lesions as well as the management of intraoperative potential complications.
Preoperative considerations
There are two main aspects that have to be addressed in these patients before anesthesia and surgery: assessment of airway compromise, and considering the need for avoiding general anesthesia while induction.
The presence of respiratory symptoms such as dyspnea, orthopnea, postural dyspnea or stridor must alert of central airway compression and the increased risk of perioperative complications (1,8), specially in severe symptoms, as shown in Table 2, and have to be addressed not only in the upright position but in different patient positions (7). Superior vena cava compression symptoms can also alarm about the vascular compression (9).
Table 2
| Imaging evidence of airway compression or displacement |
| Anterior location of the tumor |
| Pericardial effusion |
| Pleural effusion |
| Histologic diagnosis of lymphoma |
| Imaging evidence of vascular compression |
| Superior vena cava syndrome (SVCS) |
Data from Gothard JW. Anesthetic considerations for patients with anterior mediastinal masses. Anesthesiol Clin 2008;26:305-14.
Preoperative respiratory function tests (RFT’s) and arterial blood gas analysis have not shown to be useful for predicting complications, but maximal inspiratory and expiratory flow volume curves might be helpful to assess the degree of compression, and its extrathoracic or intrathoracic location (10,11). Intrathoracic airway obstruction is accompanied by a widening of the mid-expiratory plateau and is thought by some researchers to predict risk for intraoperative airway compromise (12). A mixed obstructive and restrictive pattern on RFT’s predicts a higher risk for postoperative respiratory complications (1). A computed tomography scan (CT) is usually mandatory in order to determine the grade of airway compression because those cases with more than 50% of tracheal compression are at a high risk for complications, reaching up to 37.5% in some series (1,2,9), specially postoperative pulmonary complications. CT also gives information about vascular or pericardial involvement to anticipate before the surgery. Pericardial effusion can be diagnosed with imaging tests, and is one of the most solid risk factors for intraoperative life-threatening complications (1).
Patients can be categorized into three categories of risk:
- Low risk: asymptomatic patients or mild symptoms but without postural symptoms neither radiological evidence of airway compression;
- Middle risk: mild or moderate postural symptoms, radiological evidence of airway compression less than 50%;
- High risk: severe postural symptoms, stridor, cyanosis, radiological evidence of airway compression more than 50%, tracheal compression with bronchial compression, pericardial effusion or superior vena cava syndrome (SVCS).
Management of airway compression during anesthesia can be controversial with different alternatives. Compressibility of airway can be increased due to lung volume reduction during anesthesia and bronchial smooth muscle relaxation (13). Muscular relaxation causes also loss of chest wall tone and disruption of the forces of active airway inspiration, thus reducing external support to the compressed airway. This relaxation also reduces the normal transpleural pressure gradient that dilates the airway (7). In addition, lung volume is reduced to 500–1,500 cc during general anesthesia, as well as functional residual capacity is reduced until 20% during general anesthesia (14). Compression of the lung by the mediastinal mass increases the risk for atelectasis and postoperative pneumonia (8). Some authors have described using tracheobronchial stenting for managing the compressed airway during surgery of huge mediastinal masses, but it seems only an extreme option for catastrophic cases. Preoperative use of corticosteroids, chemotherapy or radiation have been described, but they can affect the result of intraoperative surgical biopsies of mediastinal masses and should be avoided unless absolutely necessary.
Preferred patient position preoperatively has to be considered because it describes the position that alleviates more their airway compression, thus kept in mind for induction of anesthesia (15). Voluntary airway control preserving spontaneous breathing is mandatory in some patients to keep the negative subatmospheric pleural pressure and avoid catastrophic airway collapse while anesthesia induction specially if muscle relaxation is performed, but in low risk cases, muscular block with positive pressure ventilation could be an alternative option.
In relation to systemic aspects to keep in mind for mediastinal masses, remember that intrathoracic goiters can show thyroid dysfunction, so thyroid function ought to be monitored and treated preoperatively. Specific considerations for miastenia gravis surgery are explained later.
Echocardiography could be advisable if pericardial effusion is present or there are symptoms of heart or great vessels compression (8), because it predicts perioperative complications.
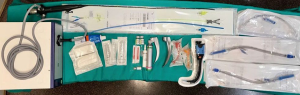
Before inducing anesthesia and starting the surgical procedure, an individualized multidisciplinary preoperative care plan should be developed and known by surgeons, anesthesiologists and nurse staff, including a table with different tools which must be set up (Figure 1), such as rigid bronchoscope, different sizes of single-lumen and double-lumen tubes for nasotracheal/orotracheal intubation, single-lumen endobronchial tubes (SLEBTs), fiberoptic bronchoscope, and material for cardiopulmonary bypass. Some authors recommend only draping femoral vessels in patients with less than 50% airway compression, but cannulation of femoral vessels under local anesthesia in patients with more than 50% of tracheal compression and specially if SVCS is present (7). Invasive arterial monitoring is mandatory in middle and high-risk patients, and a large bore intravenous access should be positioned in a lower limb if there is preoperative evidence of SVCS.
Intraoperative management
Patient position
Although the most common approaches for mediastinal masses surgery were the median sternotomy and the posterolateral thoracotomy, the advent of minimally invasive surgery has changed the way these procedures are performed. Most anterior mediastinal lesions are approached by thoracoscopic approach in 30–45 degrees oblique supine decubitus. This position is helpful for anterior lesions because it can be safely and quickly converted to supine decubitus without difficulty should cardiovascular or respiratory collapse appears (16). Supine decubitus is still the standard position for median sternotomy, but also used in subxiphoid uniportal VATS or robotic retrosternal procedures. Lateral decubitus with uniportal or multiportal VATS approach is commonly used for lesions located in the middle or posterior mediastinum, such as paragangliomas, ectopic parathyroid adenomas, neurogenic tumors or cystic lesions from different origins.
Spontaneous ventilation
An effort should be done in high-risk patients for preserving spontaneous ventilation achieving the desired level of anesthesia to decrease the probability of intraoperative complications while induction, as main case reports and case series have described (8). In low risk patients without severe symptoms, general anesthesia with neuromuscular blockade and positive pressure ventilation can be attempted safely.
Intraoperative airway management
The most common method for airway management during the procedure, is using a reinforced endotracheal tube beyond the tracheal compression, preferable by awake nasotracheal or orotracheal intubation under local anesthesia and sedation (9) and using a bronchial blocker for lung isolation (exchange to double lumen tubes can be done with airway exchange catheters). The alpha 2 agonist dexmedetomidine, and ketamine have proved to be useful due to their safe profile, with analgesic and sedative properties but low risk of respiratory depression (8). For cases with distal trachea or mainstem bronchial compression, rigid bronchoscopy and jet ventilation could be required. The use of fiberoptic bronchoscope guidance could help in placing an endobronchial tube. In this case, SLEBTs can safely secure bronchial ventilation with long tubes in the least obstructed main bronchus (7), so oxygenation can be assured. If these maneuvers fail, then cardiopulmonary bypass should be instituted to avoid cardiovascular collapse (15), prior to the need for surgical debulking of huge mediastinal masses through sternotomy or thoracotomy or holding the lesion upwards. These measures are resumed in Table 3.
Table 3
| Patient position |
| ▪ Induction in sitting position |
| ▪ Change supine position to lateral or prone position |
| Preserve spontaneous breathing |
| ▪ Awake fiberbronchoscopic intubation |
| ▪ Inhalational induction |
| ▪ Intravenous induction (ketamine, dexmedetomidine) |
| Airway intubation |
| ▪ Long endotracheal tube |
| ▪ Double-lumen endobronchial tube |
| ▪ Rigid bronchoscope |
| ▪ Tracheobronchial stents |
| Cardiopulmonary bypass |
| ▪ Started under local anesthesia before induction |
| ▪ Vessels prepared under local anesthesia, then general anesthesia |
Data from Gothard JW. Anesthetic considerations for patients with anterior mediastinal masses. Anesthesiol Clin 2008;26:305-14.
Management of intraoperative complications
Should an intraoperative complications occurs, some measures can be life-saving. If severe hypotension appears, patient should be placed in the security position considered preoperatively. Mild or moderate decrease in cardiac output should be managed with volume expansion, vasopressors and inotropic, as normal. If these measures fail, open emergent approach to elevate/hold the mediastinal mass and release the vascular//heart compression can be necessary. Cardiopulmonary bypass can be set up emergently if necessary due to cardiovascular or respiratory collapse (9), and also if SVCS is present (2).
A security position should have been decided before embarking the procedure, depending on the anatomical relationships between the mass and vital organs. The most common ones are the upright seated, lateral decubitus or prone position.
Secondary to turbulence in gas flow distal to severe airway compression, respiratory gas flow can decrease. Some authors have described the use of Heliox (mixture of helium and oxygen), because it can improve patient’s symptoms, facilitate induction of general anesthesia and it presents a better profile for ventilation weaning during the postoperative period. For this purpose, high helium fractions are mandatory, thus reducing oxygen fractions and endangering oxygenation in patients with respiratory compromise due to their airway compression (8).
Because of the proximity of mediastinal masses to phrenic and laryngeal recurrent nerves, special attention has to be kept in mind to avoid nerve injury and potential catastrophic consequences such as diaphragm or vocal cord palsy (2).
Vascular bleeding can be predicted by preoperative imaging tests, and conventional maneuvers for vascular accidental breaching can be done. If a main vessel invasion is predicted (superior vena cava, brachiocephalic vein/artery), a large intravenous cannula in the femoral vein should be placed before starting the procedure, for rapid blood transfusion should an accident occurs, and cardiopulmonary bypass has to be considered too (2).
Anesthesia for non-intubated mediastinal procedures
As mentioned in previous publications, there are some considerations that the anesthesiologist should be friendly with before initiating non-intubated mediastinal procedures (17), because it is a real challenge for the anesthesiologist specially for big masses with airway or heart compression. In addition to the well-known concerns of pulmonary non-intubated procedures, mediastinal masses increase the risk for intraoperative or postoperative complications, so special attention has to be highlighted.
Authors have already described the consecutive phases of anesthesia (18-20), and their main considerations are shown as follows:
- Arrival of the patient to the operation room: the patient must be explained again the non-intubated VATS mediastinal procedure, specially about the possibility of feeling some degree of dyspnea in case he/she is not deeply sedated in some part of the procedure.
- Standard monitoring including: electrocardiogram, oxygen saturation measured by pulse-oximetry, peripheral venous access, respiratory rate plus invasive blood pressure monitoring using the radial artery (Figure 2). The anesthetic depth is monitored through bispectral index value (BIS), and usually kept between 40 and 60, although some expertise groups have described BIS values between 30 and 50. Urinary catheter is placed in some cases, although predicted short procedures can be managed through a tubeless approach.
- For premedication we normally use between 1–3 mg of midazolam (better avoid benzodiazepines for miastenia gravis surgery) and 50–100 μg of fentanyl.
- Our common analgesia techniques are either multiple intercostal blocks at the beginning and the end of the surgery, or epidural block (as in an intubated VATS). We explain later these possibilities.
- Sedation: this is a matter of discussion, there is not a perfect drug, all of them have secondary effects. In our experience the use of a propofol infusion (2–4 mg/kg/h) plus the regional block is a good combination. Adding opioids as fentanyl (50–100 µg) is reserved to control the respiratory rate. Remifentanyl infusions are associated with severe cases of hypercapnia (PaCO2 >100 mmHg). Dexmedetomidine is a theoretical drug with a better profile but more experience should be addressed first in non-intubated pulmonary and mediastinal surgery.
- Monitors of respiratory parameters: respiratory rate must be monitored to prevent risk of hypercapnia. If the patient has a low respiratory rate, it is normally associated with deep mediastinal movement (higher surgical difficulties). Adjusting the correct balance between stable surgical field avoiding respiratory depression is sometimes one of the main challenges of this approach.
- Awakening: once the drainage is positioned and the wound is closed, the propofol infusion is stopped so the patient completely awakes and is taken to the recovery room, thus oral intake and walking begin within the next 6 hours.
- Locoregional anesthesia: non-intubated uniportal VATS mediastinal procedures can be performed under different locoregional anesthesia techniques, but the most commonly used are epidural anesthesia, intercostal blocks or local anesthesia (20). For epidural anesthesia, a catheter is placed between T4–5 and after initial administration of bupivacaine 0.5% with adrenaline, then 4–5 cc bolus ropivacaine 0.375% are infused to achieve the blockade between T2 and T9. It seems a more invasive technique when compared to selective unilateral intercostal block under direct view, with exceptional episodes of urinary retention or constipation. Before the skin incision/s, local lidocaine 2% is applied to diminish the risk of triggering pain stimuli. We routinely perform intercostal blocks in the intercostal spaces affected by the VATS incision/ports (21), with Bupivacaine 0.5%, 1–1.5 cc in each space at the beginning of the procedure, and at the end of the procedure (before closing the incision). The intercostal selective blockade can be tailored depending on the location of the thoracoscopic port sites and the use of chest tube. There are many variants of intercostal block. Initially we performed the block with an intramuscular needle from the back of the patient in order to keep the parietal pleura intact, but it can be troublesome in obese patients. Then we changed our technique and performed the block under direct thoracoscopic view with an irrigation system with a fine gauge (Figure 3). Intercostal block results a selective unilateral block, without systemic adverse events (urinary retention, constipation) and the duration of the analgesia extends within the first 24 hours. Other locoregional techniques have been described, such as paravertebral catheters, anterior serratus blockades (23), spine erector plane blockade (24), but they have been less described in non-intubated series.
 Figure 3 Intercostal multiple block with local anesthesia with direct thoracoscopic view (22). Available online: http://www.asvide.com/article/view/25875
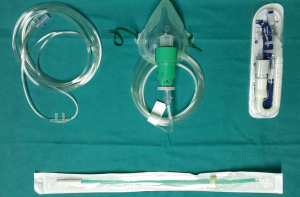
Figure 3 Intercostal multiple block with local anesthesia with direct thoracoscopic view (22). Available online: http://www.asvide.com/article/view/25875 - Oxygenation: despite these patients keep spontaneous breathing, they usually require some support for oxygenation consequence of the surgical pneumothorax and the “re-breathing” of exhaled air. There are several devices available including the laryngeal mask, but our anesthesiology team mainly prefers (Figure 4): nasopharyngeal cannula, facial mask and high-flow oxygen nasal prongs. Nasopharyngeal cannula with high FiO2 minimizes the risk of hypoxemia, and can be easily positioned with intranasal local anesthesia. Facial mask with Guedel cannula or high-flow oxygen nasal prongs have the same purpose but in our opinion are less appropriate than nasopharyngeal cannula whose tip lies immediately above the vocal cords and increases oxygen concentration in inspirated air. Oxygenation can be ensured through these techniques, but main concerns focus on the risk of hypercapnia. Secondary to “re-breathing” and deep sedation, moderate hypercapnia is usually observed. In case of severe hypercapnia we reduce the propofol infusion and we assist the patient with the facial mask until recovering normal parameters, but if severe hypercapnia remains, conversion to tracheal intubation and mechanical ventilation should be considered, specially in patients with some degree of pulmonary compromise, but can result in a challenge for the anesthesiologist due to the central compression.
- Vagal block: cough reflex is one of the challenges when facing non-intubated procedures, specially when pulling lung parenchyma is needed, being less common in mediastinal procedures. If the surgeons notice that cough is being triggered by surgical maneuvers, vagal block can be easily performed with Bupivacaine 0.5%, 2–3 cc. We inject the local anesthetic in the lower paratracheal area or in the aortopulmonary window, for right and left procedures respectively (Figure 5). Left vagal block is better performed pulling gently the lung anteriorly and incising the mediastinal pleura just after the laryngeal recurrent nerve visualization. Vagal block ensures cough abolition during 12 hours so most procedures can be safely performed. It is better to block the vagal transmission before initiating surgical maneuvers in order to avoid cough reflex triggering. Some other techniques have been described for cough control, such as intravenous or aerosolized local anesthetic, but they are less effective than direct vagal block.
 Figure 5 Vagal block with local anesthesia, performed in the right lower paratracheal area or in the left aortopulmonary window. Bupivacaine 0.5% is commonly used due to the duration of the effect (25). Available online: http://www.asvide.com/article/view/25876
Figure 5 Vagal block with local anesthesia, performed in the right lower paratracheal area or in the left aortopulmonary window. Bupivacaine 0.5% is commonly used due to the duration of the effect (25). Available online: http://www.asvide.com/article/view/25876
Anesthesia for miastenia gravis: specific considerations
First, as an autoimmune disease, miastenia gravis (MG) can coexist with other autoimmune diseases, and it’s specially remarkable that hypothyroidism and malnutrition, if detected, have to be treated before surgery (26). Other conditions and the use of some drugs can worsen the situation of myasthenic patients; specially remarkable are corticosteroids which can trigger an exacerbation of miastenia at the initial phase of treatment.
Patients with miastenia gravis can present mainly two kinds of crisis: myasthenic crisis and cholinergic crisis. Myasthenic crisis are exacerbations of the disease, usually caused by interaction of factors such as infections, emotional stress or surgery, and its prevalence is between 8–27%, being the main cause of miastenia gravis death (27). One third of the patients with miastenia gravis that experience a myasthenic crisis will suffer a repeated crisis within his lifetime. Cholinergic crisis is secondary to excessive treatment with anti cholinesterase agents, with typical cholinergic symptoms (excessive salivation, sweating, muscle weakness, bradycardia). These two types of crisis can appear with similar clinical findings, so edrophonium test can solve the cause: a single dose of edrophonium should improve a myasthenic crisis but worsen a cholinergic crisis. As aforementioned, patients that undergo mediastinal surgery are at a higher risk for myasthenic c crisis due to their own underlying disease and their hypersensitivity to neuromuscular blockade agents (28).
If a myasthenic crisis is suspected before surgery, patient has to be managed in an intensive care unit, with mobilization, respiratory support, infection prevention/treatment and monitoring of vital functions. Intravenous immunoglobulin and plasma exchange are options if immunosuppressive therapy has failed (mainly corticosteroids, but also azathioprine, cyclophosphamide, cyclosporine A and rituximab) (29), but however, prophylactic steroid therapy poses a certain risk of initial exacerbation or infection, and no standard protocols for administering plasma exchange or intravenous immunoglobulin are available (30).
Cholinergic crisis usually requires cessation of anticholinesterase therapies, atropine and sometimes intubation.
Surgery for miastenia gravis is usually reserved for stable phases of the disease, when minimal pharmacological doses are needed, and it is advisable that experienced anesthesiologists assess preoperatively the patient. Postoperative myasthenic crisis (POMC) are defined as the need for prolonged ventilator support >24–48 h after the surgery, or repeated ventilator support (including non-invasive ventilation) after extubation before postoperative day 30, with a prevalence between 6% and 34% of thymectomies. Predictive factors for POMC in a case series report from 2015 were pretreatment and preoperative AchR Ab titres, unstable miastenia and history of myasthenic crisis (30), with the last two factors being independent predictive factors in multivariate analysis. A paper from 2014 reported a new score for predicting POMC, and showed that patients with score <2.0 have less than 10% risk of crisis, whereas score >4.0 have 50% risk of a postoperative crisis (31).
Pyridostigmine maintenance until the morning of surgery is accepted nowadays for facilitating postoperative neuromuscular recovery (32,33). Routine premedication with sedative or opioids should be avoided because of the risk of respiratory depression. Succinylcholine is not recommended, but short-acting agents such as propofol, fentanyl, remifentanyl, sevoflurane and desflurane have been safely used (34).
Due to the shift in surgical approach for thymectomy from open median sternotomies to thoracoscopic approaches, one-lung ventilation to isolate the lung on the operating pleural cavity has become almost mandatory. In addition, the extended use of double-lumen tubes needs a more relaxed patient that conventional endotracheal tubes, so the use of neuromuscular blocking agents (NMBA) becomes more recommendable (35). The use of these agents has been discouraged for years, and successful anesthetic management has been described up to 71.1% of cases without usage of NMBA (36). Short action NMBA such as mivacurium in reduced doses has proved to be safe in the management of these patients, with optimal muscle relaxation and adequate early extubation. Middle action agents such as rocuronium (0.3 mg/kg), cisatracurium and vecuronium with different fractions of standard doses for healthy patients have shown a safe profile but with an increased risk of prolonged recovery (36).
Sugammadex is a selective NMBA binding agent for reversing action of steroidal NMBA rocuronium and vecuronium, by decreasing the number of free molecules available. It has been effectively used for reversing low dosis of rocuronium (2 mg/kg) (35) producing early recover after the surgery (mean time 111 sec., but there are some controversial papers about its effectiveness in reversing NMBA in patients with miastenia gravis (37,38).
Neuromuscular monitoring (TOF) is essential for adequate muscle relaxation and postoperative safe recovery (TOF >0.9) independent of NMBA utilization. While operating, if TOF ratio is >25% or diaphragm movement is observed, a supplemental dose of rocuronium can be administered, usually one fourth of initial dose. After administering sugammadex at the end of the procedure, if TOF response (time to T4/T1) does not increase higher than 0.9, patient can be taken to the recovery area or ICU intubated, awaiting for spontaneous recovery of neuromuscular blockade.
Conclusions
Mediastinal diseases requiring surgical diagnostic and/or therapeutic procedures are a heterogeneous group, but due to their location they can all threaten patient’s life during anesthesia or surgery because of the risk of cardiovascular or respiratory collapse.
Some preoperative considerations have to be kept in mind regarding patient’s symptoms and imaging evidence of vital organs compression, after categorizing patients into low, middle or high risk for perioperative complications.
Management of the airway in these patients can be challenging, so different strategies must be assessed before initiating the induction of anesthesia including rigid bronchoscope jet ventilation and starting cardiopulmonary by-pass if necessary.
Although non-intubated procedures are becoming more frequent for pulmonary surgical procedures, there is still a lack of evidence of its advantages and disadvantages in mediastinal surgery, so special care must be taken before embarking in this novel approach.
Acknowledgments
To our experts anesthesiologists in thoracic surgery for their instruction, dedication and professionalism. To the nursing surgical staff of our unit for their cooperation and support in these innovative minimally invasive and minimally aggressive procedures.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Tommaso Claudio Mineo) for the series “Mediastinal Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.05.26). The series “Mediastinal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Béchard P, Létourneau L, Lacasse Y, et al. Perioperative cardiorespiratory complications in adults with mediastinal mass: incidence and risk factors. Anesthesiology 2004;100:826-34; discussion 5A.
- Gothard JW. Anesthetic considerations for patients with anterior mediastinal masses. Anesthesiol Clin 2008;26:305-14. [Crossref] [PubMed]
- Victory RA, Casey W, Doherty P, et al. Cardiac and respiratory complications of mediastinal lymphomas. Anaesth Intensive Care 1993;21:366-9. [PubMed]
- Hammer GB. Anaesthetic management for the child with a mediastinal mass. Paediatr Anaesth 2004;14:95-7. [Crossref] [PubMed]
- Pullerits J, Holzman R. Anaesthesia for patients with mediastinal masses. Can J Anaesth 1989;36:681-8. [Crossref] [PubMed]
- Ng A, Bennett J, Bromley P, et al. Anaesthetic outcome and predictive risk factors in children with mediastinal tumours. Pediatr Blood Cancer 2007;48:160-4. [Crossref] [PubMed]
- Goh MH, Liu XY, Goh YS. Anterior mediastinal masses: an anaesthetic challenge. Anaesthesia 1999;54:670-4. [Crossref] [PubMed]
- Blank RS, de Souza DG. Anesthetic management of patients with an anterior mediastinal mass: continuing professional development. Can J Anaesth 2011;58:853-9, 860-7. [Crossref] [PubMed]
- Suarez L, Moreno I, Leyra F, et al. Anesthesia and perioperative cares for mediastinal surgery and masses. Treat of anesthesia and perioperative medicine in Thoracic Surgery, Ed Ergon, 2nd edition, 2017:215-9.
- Prakash UB, Abel MD, Hubmayer RD. Mediastinal mass and tracheal obstruction during general anesthesia. Mayo Clinic Proceedings 1988;63:1004-11. [Crossref] [PubMed]
- Miller RD, Hyatt RE. Obstructing lesions of the larynx and trachea: clinical and physiologic characteristics. Mayo Clin Proc 1969;44:145-61. [PubMed]
- Erdös G, Tzanova I. Perioperative anaesthetic management of mediastinal mass in adults. Eur J Anaesthesiol 2009;26:627-32. [Crossref] [PubMed]
- Neuman GG, Weingarten AE, Abramowitz RM, et al. The anesthetic management of the patient with an anterior mediastinal mass. Anesthesiology 1984;60:144-7. [Crossref] [PubMed]
- Wahba RW. Perioperative functional residual capacity. Can J Anaesth 1991;38:384-400. [Crossref] [PubMed]
- Sulen N, Petani B, Bacić I, et al. Anesthetic management of a patient with central airway compression due to posterior mediastinal mass. Acta Clin Croat 2016;55:103-7. [PubMed]
- Mineo TC, Tacconi F. From "awake" to "monitored anesthesia care" thoracic surgery: A 15 year evolution. Thorac Cancer 2014;5:1-13. [Crossref] [PubMed]
- Navarro-Martínez J, Gálvez C, Rivera-cogollos MJ, et al. Intraoperative crisis resource management during a non-intubated video-assisted thoracoscopic surgery. Ann Transl Med 2015;3:111. [PubMed]
- Galvez C, Navarro-Martinez J, Bolufer S, et al. Nonintubated uniportal VATS pulmonary anatomical resections. J Vis Surg 2017;3:120. [Crossref] [PubMed]
- Galvez C, Bolufer S, Navarro-martinez J, et al. Awake uniportal video-assisted thoracoscopic metastasectomy after a nasopharyngeal carcinoma. J Thorac Cardiovasc Surg 2014;147:e24-6. [Crossref] [PubMed]
- Galvez C, Navarro-martinez J, Bolufer S, et al. Non-intubated uniportal left-lower lobe upper segmentectomy (S6). J Vis Surg 2017;3:48. [Crossref] [PubMed]
- Kiss G, Castillo M. Nonintubated anesthesia in thoracic surgery : general issues. Ann Transl Med 2015;3:110. [PubMed]
- Galvez C, Galiana M, Corcoles JM, et al. Intercostal multiple block with local anesthesia with direct thoracoscopic view. Asvide 2018;5:620. Available online: http://www.asvide.com/article/view/25875
- Font MC, Navarro-Martinez J, Nadal SB, et al. Continuous Analgesia Using a Multi-Holed Catheter in Serratus Plane for Thoracic Surgery. Pain Physician 2016;19:E684-6. [PubMed]
- Adhikary SD, Pruett A, Forero M, et al. Erector spinae plane block as an alternative to epidural analgesia for post-operative analgesia following video-assisted thoracoscopic surgery: A case study and a literature review on the spread of local anaesthetic in the erector spinae plane. Indian J Anaesth 2018;62:75-8. [Crossref] [PubMed]
- Galvez C, Galiana M, Corcoles JM, et al. Vagal block with local anesthesia, performed in the right lower paratracheal area or in the left aortopulmonary window. Bupivacaine 0.5% is commonly used due to the duration of the effect. Asvide 2018;5:621. Available online: http://www.asvide.com/article/view/25876
- Sungur Z, Sentürk M. Anaesthesia for thymectomy in adult and juvenile myasthenic patients. Curr Opin Anaesthesiol 2016;29:14-9. [Crossref] [PubMed]
- Alshekhlee A, Miles JD, Katirji B, Preston DC, Kaminski HJ. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology 2009;72:1548-54. [Crossref] [PubMed]
- Korst RJ. Take a deep breath but don't relax: Anesthesia for thymectomy in myasthenia gravis. J Thorac Cardiovasc Surg 2018;155:1890. [Crossref] [PubMed]
- Barth D, Nabavi N, Ng E, et al. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011;76:2017-23. [Crossref] [PubMed]
- Ando T, Omasa M, Kondo T, et al. Predictive factors of myasthenic crisis after extended thymectomy for patients with myasthenia gravis. Eur J Cardiothorac Surg 2015;48:705-9; discussion 709. [Crossref] [PubMed]
- Leuzzi G, Meacci E, Cusumano G, et al. Thymectomy in myasthenia gravis: proposal for a predictive score of postoperative myasthenic crisis. Eur J Cardiothorac Surg 2014;45:e76-88. [Crossref] [PubMed]
- Tripathi M, Kaushik S, Dubey P. The effect of use of pyridostigmine and requirement of vecuronium in patients with myasthenia gravis. J Postgrad Med 2003;49:311-4; discussion 314-5. [PubMed]
- Sungur Ulke Z, Senturk M. Mivacurium in patients with myasthenia gravis undergoing video-assisted thoracoscopic thymectomy. Br J Anaesth 2009;103:310-1. [Crossref] [PubMed]
- Gritti P, Sgarzi M, Carrara B, et al. A standardized protocol for the perioperative management of myasthenia gravis patients. Experience with 110 patients. Acta Anaesthesiol Scand 2012;56:66-75. [Crossref] [PubMed]
- Sungur Ulke Z, Yavru A, Camci E, et al. Rocuronium and sugammadex in patients with myasthenia gravis undergoing thymectomy. Acta Anaesthesiol Scand 2013;57:745-8. [Crossref] [PubMed]
- Fujita Y, Moriyama S, Aoki S, et al. Estimation of the success rate of anesthetic management for thymectomy in patients with myasthenia gravis treated without muscle relaxants: a retrospective observational cohort study. J Anesth 2015;29:794-7. [Crossref] [PubMed]
- Sugi Y, Nitahara K, Shiroshita T, et al. Restoration of train-of-four ratio with neostigmine after insufficient recovery with sugammadex in a patient with myasthenia gravis. A A Case Rep 2013;1:43-5. [Crossref] [PubMed]
- Kiss G, Lacour A, d’Hollander A. Fade of train-of-four ratio despite administration of more than 12mgkg(-1) sugammadex in a myasthenia gravis patient receiving rocuronium. Br J Anaesth 2013;110:854-5. [Crossref] [PubMed]
Cite this article as: Galvez C, Galiana M, Corcoles JM, Lirio F, Sesma J, Bolufer S. Current anesthesiological approach to mediastinal surgery. J Vis Surg 2018;4:146.