
Mediastinal silicon-induced lymphadenopathy mimicking “N3” disease in resectable lung cancer
Introduction
Since the first silicone mammary implant in the early 60’s, the use of silicone breast prostheses for reconstructive and cosmetic purposes has grown considerably (1). Silicone implant leakage is associated with several complications, among which mediastinal silicon-induced lymphadenitis have been previously reported (2).
Although isolated silicone lymphadenopathy does not represent—most of the time—a clinical issue, being asymptomatic and not requiring specific treatment, it may raise concern for malignancy or metastatic cancer, in particular in patients with a past history of breast cancer or a present diagnosis of primary tumors of other organs.
Clinical assessment by computed tomography (CT) and positron emission tomography (PET) scan can be misleading, disclosing enlarged lymph nodes with high [18F]-FDG uptake values, thus mimicking metastatic lymphadenopathy.
Here we report a case of a patient with a pathological diagnosis of primary lung cancer and clinical contralateral mediastinal involvement—a stage IIIB cN3 disease—potentially excluding from curative resection, in which thoracoscopic excision of the lymphadenopathy showed a silicon-induced lymphadenitis, thus offering the patient the best therapeutic option for lung cancer.
Case presentation
A 62-year-old former smoker woman—with a past history of bilateral breast cancer and melanoma—had a chest CT during routine oncological follow-up. Twenty and three years earlier she underwent left and then right mastectomy for mucinous breast carcinoma and ductal infiltrating carcinoma respectively. Mono-lateral left breast reconstruction was then performed with silicone prosthesis. She then underwent chemotherapy, hormonal treatment and radiotherapy.
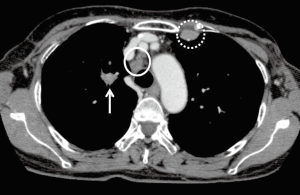
A primary adenocarcinoma of the right upper lobe with ipsilateral mediastinal involvement (pN2 disease) was diagnosed and the patient was submitted to induction treatment by cisplatin and gemcitabine. Re-staging by CT (Figure 1) and PET-scans disclosed a decrease in size and pathological FDG-uptake of both the right upper lobe nodule and ipsilateral mediastinal lymphadenopathies; on the contrary, a swelling and increase of FDG uptake of the left internal mammary chain lymph node was also detected, thus suggesting a progressive N3 disease, not amenable of curative lung resection. Due to the uncommon site of mediastinal lung cancer metastases and to the discrepancy between reducing N2 lymph nodes and tumor with suspected progressive N3 lymph nodes, we then decided to biopsy the increasing lymph node of the left internal mammary chain. Because of the close proximity of the target lesion to internal mammary vessels and the presence of breast prosthesis, CT or ultrasound (US) guided biopsy was skipped and the patient underwent left video-assisted thoracoscopic (VAT) excisional biopsy of the internal mammary lymphadenopathy (Figure 2).

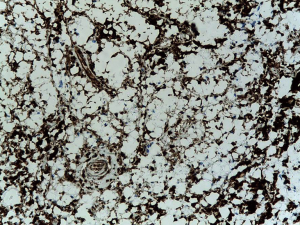
Definitive histology disclosed distortion of the lymph node architecture by histiocytes with vacuolated and clear cytoplasm containing silicone compounds, diagnostic for mediastinal silicon-induced lymphadenitis due to previously unknown breast prosthesis rupture (Figure 3).
Comments
According to the VIII edition of the TNM staging system, contralateral mediastinal involvement in primary lung cancer is staged as IIIB N3 disease and patients in this stage are excluded form surgical resection as curative option (4). For this reason, an accurate mediastinal staging is of paramount importance in lung cancer: mediastinoscopy—in the past—and endobronchial ultrasound trans bronchial needle aspiration (EBUS-TBNA) during flexible bronchoscopy—at the present—represent the standard tools for lymph node biopsies, thus overcoming false positive findings of clinical staging by CT and PET scan (5,6). However, internal mammary chains lymph nodes cannot be reached neither by mediastinoscopy nor by EBUS-TBNA; for this reason, VAT approach is preferred when excisional biopsy of the suspected lymphadenopathy is required. Silicon-induced lymphadenitis should always be considered—in case of internal mammary chain lymphadenopathy in patients with breast implants—as potential differential diagnosis with lymph nodal metastases. Considering that since the introduction of the first silicone breast prosthesis, more than 240 different types and 8,300 models of breast implants and expanders have been manufactured in the United States alone, this misleading finding should not be considered as anecdotal (7).
Silicone lymphadenopathy is due to the deposition, in one or more lymph nodes, of migrated silicone through tissues after clear damage of a silicone-containing membrane or through a continued slow bleed of gel through an intact surface. Once outside the confines of the prosthesis, silicone migrates to regional lymphatic stations through macrophages in the reticuloendothelial system. Subsequently, a granulomatous reaction to the foreign body causes lymph nodes enlargement, posing diagnostic challenges, as cancer is often the initial consideration, even because of CT and PET positive findings (8).
In conclusion, the chance of silicone lymphadenopathy should be considered within the differential diagnosis for patients with a proven diagnosis of cancer and mammary implants; lymph node biopsy is suggested and the assessment of leakage or rupture of the implant should be taken into consideration.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.07.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ryu AJ, Glazebrook KN, Samreen N, et al. Spectrum of Chronic Complications Related to Silicone Leakage and Migration. Am J Med 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Petrella F, Pruneri GC, Ghioni M, et al. Synchronous primary lung cancer, breast cancer recurrence, and mediastinal silicon-induced lymphadenitis J Thorac Oncol 2010;5:560-1. [Crossref] [PubMed]
- Petrella F, Prisciandaro E, Mariolo AV, et al. Internal mammary chain VAT lymphadenectomy. Asvide 2018;5:617. Available online: http://www.asvide.com/article/view/25841
- Detterbeck FC, Chansky K, Groome P, et al. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1433-46. [Crossref] [PubMed]
- Guarize J, Casiraghi M, Donghi S, et al. Endobronchial Ultrasound Transbronchial Needle Aspiration in Thoracic Diseases: Much More than Mediastinal Staging. Can Respir J 2018;2018:4269798 [Crossref] [PubMed]
- Guarize J, Casiraghi M, Donghi S, et al. EBUS-TBNA in PET-positive lymphadenopathies in treated cancer patients. ERJ Open Res 2017;3. [PubMed]
- Zambacos GJ, Molnar C, Mandrekas AD. Silicone lymphadenopathy after breast augmentation: case reports, review of the literature, and current thoughts. Aesthetic Plast Surg 2013;37:278-89. [Crossref] [PubMed]
- Gilbert LK, Thiruchelvam J. Cervical silicone lymphadenopathy. Br J Oral Maxillofac Surg 2016;54:e52-4. [Crossref] [PubMed]
Cite this article as: Petrella F, Prisciandaro E, Mariolo AV, Girelli L, Pirola S, Spaggiari L. Mediastinal silicon-induced lymphadenopathy mimicking “N3” disease in resectable lung cancer. J Vis Surg 2018;4:144.


