
Inferior vena cava resection with prosthesis replacement for recurrent leiomyosarcoma (with video)
Introduction
Primary malignant tumor of the inferior vena cava (IVC) is rare and seldom asymptomatic, it concerns mostly middle aged women (1) in the 2nd or middle segment of the IVC (2), between hepatic and renal veins according to Kulaylat’ classification (3), and recurs in 33% of patients (4). Leiomyosarcoma (LY) of the IVC arises from smooth muscle cells of caval intima without any clearly identified predisposing risk factors (5). Surgical resection is the mainstay of therapy, with or without venous reconstruction, providing a chance for long term survival and good functional outcomes. Patients undergoing complete resection have a 3- and 5-year disease-specific survival rates of 76% and 33%, respectively (4). Herein the case report of a 76 years old lady.
Case presentation
An asymptomatic 76-year-old woman came to our attention for a recurrent IVC LY. The patient underwent 10 years before a partial caval wall resection with reconstruction using a patch of pericardium bovine for a low-grade LY, out of follow-up. Her medical history reported only a hysteroannessectomy due to fibromatosis and a QUART for left breast cancer 2 years earlier followed yearly since then. On her annual liver ultrasound, a mass of 45 mm in the retroperitoneum was highlighted. The following CT-scan showed a lesion of 50×48×30 mm arising from the segment II of IVC, with inhomogeneous contrast enhancement suggestive for recurrent caval sarcoma that extended from retrohepatic IVC to left renal vein with no involvement of hepatic veins (Figure 1). Furthermore, an area of inhomogeneous liver parenchyma measuring 20×18 mm was highlighted in the left lobe causing biliary compression, highly suggestive for metastases.
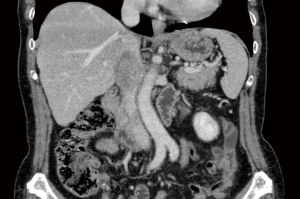
A multidisciplinary discussion with oncologists, pathologists, radiotherapists and general surgeons leaded to surgical intervention.
The patient underwent a caval resection with upper right and left renal vein ligation and reconstruction with polyethylene terephthalate (PET) prosthesis replacement. A synchronous left lateral liver resection was associated.
Postoperatively the patient developed a renal insufficiency requiring temporary dialytic treatment.
The pathological report showed a recurrent LY of 5.5 cm (pT2b) with G2 differentiation and desmin, CD34 and smooth muscle actine expression. There were 4 mitosis/10 HPF and ki67 was 15–20%. One retrieved node was free from neoplastic infiltration (pN0). The left lobe of the liver was significant for focal nodular hyperplasia.
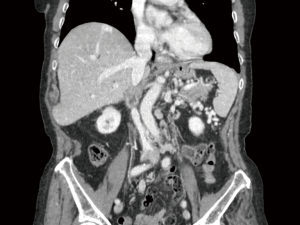
At 24 months follow up the patients is alive and free of recurrence, with a normal renal function although caval prosthesis has clotted and IVC has no residual flow (Figure 2). The patient developed several peripheral circular vascular bed and no evidence of lower limbs oedema (Figure 3).

Discussion
LY of IVC is a rare disease, surgical treatment (2,4,7,8) with negative margins is the treatment of choice, there is no evidence that preoperative radiation therapy allows a negative resection margin.
IVC reconstruction is possible (5,7,8) but there are several series reporting no reconstruction and prosthesis occlusion (4,5,8,9).
IVC could be managed with ligation with a low risk of severe postoperative edema probably due to the developing of peripheral vascular bed during the tumor growth (4,9,10).
Lower limb edema is often transitory and well tolerated for this is important to preserve collateral venous drainage.
In our department we experienced two cases, the first one (10) was treated by IVC ligation and in the second one, here reported, the concern regarding the venous drainage after the previous gynecologic procedure brought us to perform a IVC reconstruction. Both cases, followed at two years, are free of disease without evidence of venous stasis.
The long-term functional results despite graft occlusion and transient renal insufficiency agree with the authors that do not consider IVC reconstruction mandatory.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.06.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wachtel H, Gupta M, Bartlett EK, et al. Outcomes after resection of leiomyosarcomas of the inferior vena cava: a pooled data analysis of 377 cases. Surg Oncol 2015;24:21-7. [Crossref] [PubMed]
- Mingoli A, Cavallaro A, Sapienza P, et al. International registry of inferior vena cava leiomyosarcoma: analysis of a world series on 218 patients. Anticancer Res 1996;16:3201-5. [PubMed]
- Kulaylat MN, Karakousis CP, Doerr RJ, et al. Leiomyosarcoma of the inferior vena cava: A clinicopathologic review and report of three cases. J Surg Oncol 1997;65:205-17. [Crossref] [PubMed]
- Hollenbeck ST, Grobmyer SR, Kent KC, et al. Surgical treatment and outcomes of patients with primary inferior vena cava leiomyosarcoma. J Am Coll Surg 2003;197:575-9. [Crossref] [PubMed]
- Illuminati G, Pizzardi G, Calio' F, et al. Outcome of inferior vena cava and noncaval venous leiomyosarcomas. Surgery 2016;159:613-20. [Crossref] [PubMed]
- Antolino L, Valabrega S, Amato S, et al. IVC resection with prosthesis replacement for recurrent leiomyosarcoma. Asvide 2018;5:600. Available online: http://www.asvide.com/article/view/25692
- Mingoli A, Sapienza P, Brachini G, et al. Surgical treatment of inferior vena cava leiomyosarcoma. J Am Coll Surg 2010;211:145-6. [Crossref] [PubMed]
- Wachtel H, Jackson BM, Bartlett EK, et al. Resection of primary leiomyosarcoma of the inferior vena cava (IVC) with reconstruction: a case series and review of the literature. J Surg Oncol 2015;111:328-33. [Crossref] [PubMed]
- Daylami R, Amiri A, Goldsmith B, et al. Inferior vena cava leiomyosarcoma: is reconstruction necessary after resection? J Am Coll Surg 2010;210:185-90. [Crossref] [PubMed]
- Nigri G, Petrucciani N, Sirimarco D, et al. Inferior vena cava resection without prosthesis replacement for vena cava sarcoma (with video). J Visc Surg 2016;153:387-8. [Crossref] [PubMed]
Cite this article as: Antolino L, Valabrega S, Amato S, Kazemi Nava A, Sirimarco D, Moschetta G, Nigri G, Aurello P, Ramacciato G, D’Angelo F. Inferior vena cava resection with prosthesis replacement for recurrent leiomyosarcoma (with video). J Vis Surg 2018;4:134.


