
The endovascular repair of the aortic arch using a double branched prosthesis
Introduction
Along with the evolution of the aortic surgery the appearance of the thoracic endovascular aortic repair (TEVAR) provided a new therapeutic approach for the patients with acute and chronic aortic pathology. Combining the surgical with the endovascular repair broadened the vision in the treatment of the aortic arch. The surgical technique of frozen elephant trunk (FET) has developed through the years and is actually an excellent approach, providing the option for endovascular and surgical extension (1,2). However, the perioperative-stroke remains in the different case series between 2.5% and 20% (3). Additionally, the supra-aortic transposition has showed to be a reliable alternative excepting the total arch rerouting, which is related with a high rate of retrograde type A dissection (4,5). Nevertheless, there are patient groups with high risk, where neither the surgical or the hybrid repair is the first choice. In such cases total endovascular arch repair with the Bolton Relay plus double-branch endoprosthesis (Bolton Medical, Sunrise, FL, USA) could be a feasible option with excellent outcome.
However, not every patient is suitable for the approach and there are some clinical geometrical and technical details which should be considered. The geometrical and clinical details guide our path through the treatment of the thoracic aortic disease involving the aortic arch, because our primary goal is providing excellent therapeutic approach with feasible long-term outcome, reducing the aortic-related events without increasing the perioperative risks. Summarized, there are two major points, that should be considered for the use of the double branches prosthesis for the total endovascular arch repair: clinical assessment and geometrical details.
Clinical assessment
Currently, the estimation if a patient is suitable or non-suitable for classical surgery remains at the discretion of the individually treating physician as there is a lack of risk prediction models in patients with thoracic aortic pathology. However, concomitant cardiac and vascular condition and/or severe chronic obstructive lung disease are known predictors of adverse outcome in classical surgery. Therefore, this group may qualify best for the total endovascular approach.
Regarding the usage of currently available risk scores, patients with higher EuroSCORE and ASA score levels (American Society of the Anesthesiologist) should be generally favored (6-8). Patients with connective tissue disease should be generally excluded. Life expectancy more than 2 years is recommended. Individuals with congestive heart failure in class III or IV should be excluded as well as patient with significant supraaortic atherosclerosis (stenosis of the internal carotid artery ≥70% by NASCET criteria) (8,9).
Geometrical details
The aortic anatomy should be evaluated with thin-sliced computed tomography of the entire aorta including the supraaortic, iliac and femoral vessels. “Shaggy aorta” due to ulcerative atherosclerosis has a very high risk of perioperative cerebrovascular embolic events and these patients might be suboptimal candidates for total endovascular arch repair. There is generally no recommendation in relation to the angulation. However, steep arches may have a weaker performance due to the risk of kinking and potential type IIIb endoleakage due to strut perforation. On the other hand, there are clearly defined needs about the diameter and length of the ascending aorta. Diameter should be 40 mm and preferably less and ascending length should be 65 mm or longer using a center-lined measurement
The brachiocephalic trunk and the left common carotid artery (LCCA) should have regular diameters and the minimum diameter of the LCCA recommended is 7 mm. The window for the supra-aortic branches is 5 cm in length and the space from the brachiocephalic trunk offspring to the end of the LCCA has to be ≤50 mm. Currently, all devices are custom-made (Table 1).
Table 1
| Geometrical details | Minimal requirements |
|---|---|
| Length from sinotubular junction to the BT | ≥65 mm |
| Diameter of the ascending aorta | ≤40 mm |
| Diameter of the LCCA | ≥7 mm |
| Space between BT and LCCA | ≤50 mm |
| Oversizing | ≤15% |
| Access vessel size | ≥8–9 mm |
Prosthesis details
The main body is delivered in a standard retrograde fashion with an outer sheath diameter of 25 French. The main body has two internal tunnels for the supra-aortic branches (LCCA—anterior tunnel, BT—posterior tunnel). The extensions are originally modified iliac limbs of the abdominal endovascular prosthesis of Bolton (Treo). The profile of the supraaortic extensions is 14 F. Oversizing more than 15% is generally not recommended (in all zones). Exception could be made by post dissection aneurysm, where the distal landing zone is sized according to the true lumen.
The self-alignment mechanism of the main body allows that the pre-curved tip adapts itself according to the curve of the aortic arch. The mounting of the supra-aortic window to the outer curvature is automatically and radiopaque markers indicate the appropriate orientation. Nevertheless, exact active positioning remains a prerequisite (Figure 1).
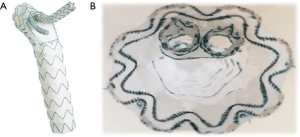
Procedure
Revascularization of the left subclavian artery (LSA) is performed before the endovascular procedure for maintaining the inflow in the left vertebral artery for the cerebellar and spinal cord perfusion. The primary goal is the reduce risk of perioperative stroke and symptomatic spinal cord ischemia (6,10). If the left vertebral artery is non-dominant, overstenting of the LSA without revascularization may be performed. Neuromonitoring with motor evoked potentials and somatosensory evoked potentials combined with cerebral fluid drainage should be used if distal TEVAR extension is planned.
After systematic heparinization (100 IU/kg) the main body is delivered and the window for the supra-aortic branches is orientated correctly according to the radiopaque markers. After inducing hypotensive conditions using rapid pacing the main body is deployed. Next step is the implantation of the supra-aortic branches and begins with the posterior tunnel, which could be deployed through the right common carotid artery or the right subclavian artery using surgical cut down. The precise cannulation of the tunnel is verified by inflating a contrast-filled balloon. The procedure is followed likewise by the insertion of the second supra-aortic branch for the LCCA (Figure 2). Finally, on table angiography is performed to prove the result (Figure 3). Mono antiplatelet therapy with 100 mg aspirin daily from postop day 1 is recommended.
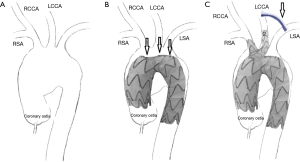
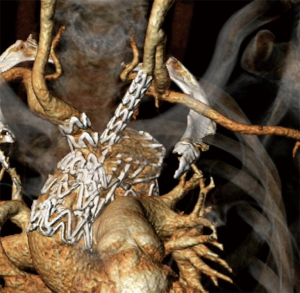
Pros and cons
The proximal landing zone is usually straight and, on this account, endoleaks type IA are rare. Zone 0 per se has a certain risk of retrograde type A dissection. However, the incidence seems to be less than in a total arch rerouting and TEVAR setting potentially due to the lack of tangential clamping of the ascending aorta (4,11). In addition, the knowledge that patients with the underlying pathology of type B aortic dissection also do have an inherently diseased ascending aorta has become accepted in the community and therefore these kinds of approaches are no longer offered to patients with this condition thereby reducing the incidence of retrograde type A aortic dissection. However, the endografts are custom made and time is needed for the manufacturing. Therefore, time per se excludes several patients from clinical implementation. Finally, there are no long-term results of the approach and the clinical experience is actually scarce.
Conclusions
Total endovascular aortic arch repair is a feasible option with an excellent aortic-related survival and very good neurological outcome. Our armamentarium in the treatment of the aortic arch has broadened with the availability of this approach. Still, there are some geometrical and clinical details, which must be taken into account when selecting patients. Nevertheless, the clinical experience with the new method is still limited and there is no data about the long-term durability of this approach. Consequently, for a potential recommendation of a broader implementation, further studies are needed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Di Bartolomeo, Davide Pacini and Mohamad Bashir) for the series “Special Edition on The 9th Postgraduate Course on ‘Surgery of The Thoracic Aorta’ in Bologna” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Special Edition on The 9th Postgraduate Course on ‘Surgery of The Thoracic Aorta’ in Bologna” was commissioned by the editorial office without any funding or sponsorship. BR and MC are consultants for Bolton Medical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Folkmann S, Weiss G, Pisarik H, et al. Thoracoabdominal aortic aneurysm repair after frozen elephant trunk procedure. Eur J Cardiothorac Surg 2015;47:115-9. [Crossref] [PubMed]
- Shrestha M, Beckmann E, Krueger H, et al. The elephant trunk is freezing: The Hannover experience. J Thorac Cardiovasc Surg 2015;149:1286-93. [Crossref] [PubMed]
- Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg 2015;47:759-69. [Crossref] [PubMed]
- Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde Ascending Aortic Dissection During or After Thoracic Aortic Stent Graft Placement: Insight From the European Registry on Endovascular Aortic Repair Complications. Circulation 2009;120:S276-81. [Crossref] [PubMed]
- Gottardi R, Funovics M, Eggers N, et al. Supra-aortic Transposition for Combined Vascular and Endovascular Repair of Aortic Arch Pathology. Ann Thorac Surg 2008;86:1524-9. [Crossref] [PubMed]
- Czerny M, Rylski B, Morlock J, et al. Orthotopic branched endovascular aortic arch repair in patients who cannot undergo classical surgery. Eur J Cardiothorac Surg 2018;53:1007-12. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. EuroIntervention 2012;8:782-95. [Crossref] [PubMed]
- Spear R, Haulon S, Ohki T, et al. Editor’s Choice - subsequent results for arch aneurysm repair with inner branched endografts. Eur J Vasc Endovasc Surg 2016;51:380-5. [Crossref] [PubMed]
- Von Reutern GM, Goertler MW, Bornstein NM, et al. Grading carotid stenosis using ultrasonic methods. Stroke 2012;43:916-21. [Crossref] [PubMed]
- Haulon S, Greenberg RK, Spear R, et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg 2014;148:1709-16. [Crossref] [PubMed]
- Czerny M, Weigang E, Sodeck G, et al. Targeting landing zone 0 by total arch rerouting and TEVAR: Midterm results of a transcontinental registry. Ann Thorac Surg 2012;94:84-9. [Crossref] [PubMed]
Cite this article as: Kondov S, Kreibich M, Rylski B, Siepe M, Beyersdorf F, Czerny M. The endovascular repair of the aortic arch using a double branched prosthesis. J Vis Surg 2018;4:132.


