Mediastinal surgery: modern treatment of primary germ cell tumor of the mediastinum
Introduction
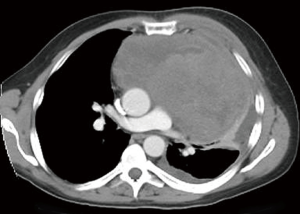
Extragonadal primary germ cell tumors (GCTs) are most commonly found in the anterior mediastinum (Figures 1,2). They may be benign (teratomas) or malignant [seminomas, nonseminomatous GCTs (NSGCTs), and mixed]. Malignant GCTs are much more common in males (1).
Patient selection
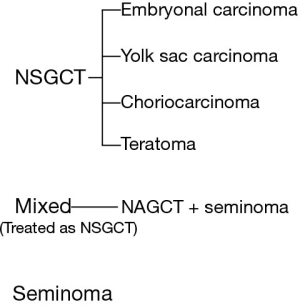
GCTs must be considered in the differential diagnosis of the anterior mediastinal mass, particularly in young males. The other most common diagnoses in the differential amongst all patient groups include thyroid tumors, thymomas, and lymphomas. Initial workup of any anterior mediastinal mass should include drawing alpha-fetoprotein (AFP), β-human chorionic gonadotropin (β-HCG), and lactate dehydrogenase (LDH) levels, along with thyroid function tests, and in appropriate cases, myasthenia gravis related antibody tests. Figure 3 shows the relationship between classifications of GCT histologies (2).
Mediastinal NSGCTs can be accompanied by hematological disease including megakaryocytic leukemia, myelodysplastic syndrome, refractory thrombocytopenia, or refractory anemia. Tumor marker elevation is seen in a majority of patients; with AFP increased in 80% of NSGCTs and β-HCG in 30% to 35%. In younger male patients, high β-HCG can lead to gynecomastia. Patients may have elevation of both AFP and β-HCG or either marker in isolation (3,4). The elevation of any nonseminomatous tumor marker, even in a tumor with predominantly seminomatous histology, is classified and treated as a NSGCT. Elevated serum AFP is not seen with pure seminomas and is an indication that yolk sac tumor and embryonal cell carcinoma is present in the primary or in a metastatic site. In mature teratomas, the markers are normal (2).
Some institutions treat any anterior mediastinal mass with β-HCG >1,000 and/or any elevation in AFP as a confirmed primary NSGCT (5). Chemotherapy with etoposide (VP-16), ifosfamide, and cisplatin (VIP) is preferred over bleomycin, etoposide, and cisplatin (BEP) to spare pulmonary complications in these patients who often require extensive surgical resection. Of note, bleomycin is toxic to the lungs. Patients with normal tumor markers should have biopsy of their mass. With a histologic diagnosis of pure seminoma and the absence of tumor marker elevation, chemotherapy with (BEP) provides an excellent prognosis without surgical resection. Mature teratoma with normal tumor markers should be surgically resected with no role for chemotherapy or radiation even in incomplete resection. Teratoma with elevated tumor markers should be treated similarly to NSGCTs with neoadjuvant VIP and potential for post-chemotherapy surgical resection.
We recommend histologic diagnosis once positive tumor markers are obtained. The pathologic discrepancy between histology and fine-needle aspiration (FNA) is 6%. Core needle biopsy or, when appropriate, surgical biopsy, typically via anterior mediastinotomy (Chamberlain procedure), will assist in establishing the diagnosis of GCT which can mimic poorly differentiated carcinoma (6). Genetic testing for the i(12p) chromosomal abnormality, a finding consistent in GCT but rarely in other tumors, may be helpful in cases with diagnostic uncertainty. Patients with rapidly declining tumor markers in the setting of platinum-based chemotherapy have improved response rates and overall survival (7). Viable tumor in the surgical specimen is an indication for further chemotherapy.
Following chemotherapeutic treatment of a NSGCT, a mass on computed tomography (CT) in the setting of normal tumor marker is best addressed by surgical resection. In the past, salvage chemotherapy was used to address continued elevation of tumor. However, this additional chemotherapy and the resulting delay in surgery has not shown any benefit to patients (8). Our current practice is to recommend surgery aimed at complete resection after initial chemotherapy despite any persistence of tumor marker elevation. Preoperative salvage chemotherapy should ideally be performed only in a clinical trial setting. In rare instances a post-chemotherapy patient will demonstrate normal tumor marker levels and no residual mass on CT. In these cases, surgery is not recommended and follow up should involve serial CT scans (2).
Preoperative preparation
Surgical assessment should occur prior to chemotherapy and again post-chemotherapy. When initial CT imaging suggests anatomic lung resection may be required, then VIP therapy is preferred over bleomycin-based chemotherapy. Surgeons should be aware of bleomycin toxicity and the associated progressive pulmonary fibrosis. In anticipation of potential lung resection, the patient’s pulmonary function, particularly their diffusion capacity (DLCO) should be measured following chemotherapy to assist in surgical planning and intraoperative decisions. The patient’s functional status and hematologic function should also be assessed (5).
Operative steps
Pre-induction
Since exposure and/or resection often requires lung collapse, the procedure is performed with lung isolation available (either double lumen endotracheal tube or bronchial blocker is acceptable). However, the fraction of inspired oxygen (FiO2) should be kept as low as possible when neoadjuvant therapy included bleomycin, as the lungs are prone to oxygen toxicity status-post bleomycin therapy. When surgery may involve resection of the superior vena cava (SVC), access should include a femoral venous line (2).
Incisions
Choosing the most appropriate incision to approach a mediastina GCT is crucial to operative success. The tumor’s size, location, and structures involved are considered in the decision. The chosen point of entry determines the obtainable views and the accessibility of structures for dissection (Figure 4). One should also be aware of the options for extension and modification of the incision should additional exposure be required.
Median sternotomy
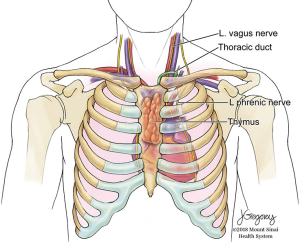
Small midline tumors in the anterior mediastinum are best approached by sternotomy. The patient is positioned supine and both arms are tucked to the sides. A shoulder roll facilitates neck extension. This positioning provides exposure of the neck, mediastinal vessels, lung hila, and hemithoraces. However, visualization and exposure of the left lower lobe and bilateral posterior lung aspects is restricted. Furthermore, when a phrenic nerve is sacrificed, plication of the diaphragm may be difficult from this incision (2).
Hemi-clamshell thoracotomies, with and without extension including trap door
The hemi-clamshell and its variants provide good exposure for large tumors of the anterior mediastinum with significant extension into either hemithorax (9). This approach combines upper median sternotomy and anterior thoracotomy (Figures 5-7). The patient is positioned supine with arms tucked at the sides. A longitudinal roll beneath the scapula is used to elevate the side of hemithorax involvement and anterior thoracotomy by about 30 degrees. Subpectoral flaps allow exposure of the intercostal space (usually the 4th) before opening this at the caudal end of the sternotomy. The mammary vessels are ligated at the level of thoracotomy. Collapse of the ipsilateral lung facilitates exposure and anatomical lung resection if necessary. The involved lung is separated from the tumor (a stapled wedge resection may be necessary) affording exposure of the posterolateral aspect of the tumor and assessment of adjacent vascular structures and phrenic nerve. In cases with neck involvement, especially when vascular dissection is required, extension of the upper sternotomy along the anterior border of the sternocleidomastoid provides excellent exposure of the carotid and jugular vessels, as well as the vagus nerve and thoracic duct on the left side. Rarely, complete removal of a mediastinal GCT will require resection of the subclavian vessels. In these cases, better control of the vessels can be obtained by extending the upper portion of the sternotomy transversely along the superior aspect of the clavicle (trapdoor). If additional exposure is required, the medial 1/3 of the clavicle may be excised (2).
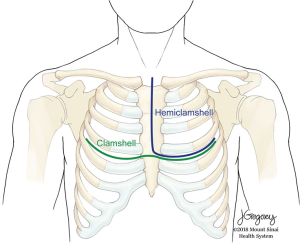
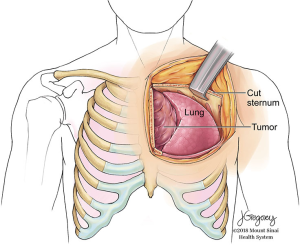
Clamshell
The clamshell incision is the best approach to very large tumors extending into both hemithoraces (10). The patient is positioned supine with both arms extended to the side or flexed and secured over the forehead. A curvilinear incision is made following the inframammary crease and extending to the right and left anterior axillary lines (Figure 5). The thorax is entered through bilateral intercostal spaces (usually the 4th) with ligation of the mammary vessels and transverse division of the sternum. Two Finochietto retractors hold separation. The pleural reflections are incised exposing the mediastinal structures. Should an initial approach with a clamshell incision yield insufficient exposure of the superior mediastinum, an upper sternal split may provide improved access to involved structures (Figure 8) (2).
Posterolateral thoracotomy
A posterolateral thoracotomy may be useful in select cases. However, this affords limited access to the mediastinal vascular structures and conversion to a median sternotomy after posterolateral dissection of the mass may be required (2).
Intraoperative decision making
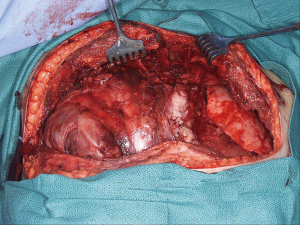
Mediastinal GCTs typically respond very well to neoadjuvant chemotherapy, but frequently the residual tumor is intimate with adjacent structures without discernible tissue planes. The decision is then left to the surgeon whether to resect major structures in consideration of related morbidity or to leave potentially viable tumor unresected. Despite distorted tissue planes, it may be possible to peel mass from certain structures such as the SVC and phrenic nerve. Peeling tissue from adjacent structures is reasonable if only fibrosis is evident, but any tumor on frozen section analysis should lead to en bloc resection when feasible.
The role of frozen section analysis is limited in these cases. Many lesions contain mixed necrotic and viable tumor. Additionally, tumors may contain teratoma, NSGCT, seminomatous, carcinomatous and sarcomatous elements. Residual tumor may be viable in 66% of cases (11). Hence intraoperative sampling may not represent the entire specimen and can provide unreliable guidance in the decision to resect or spare a major structure. Because of the limitations of biopsy and high incidence of residual viable tumor, we recommend en bloc resection of technically resectable structures whenever invasion is suspected.
We maintain a low threshold for en bloc removal of these resectable structures including the lung, pericardium, and SVC. To assess whether complete resection is possible and warrants a more radical procedure, dissection usually proceeds from left to right since unresectable structures such as the aorta are generally found to the left of resectable structures such as the SVC. When leaving residual disease is unavoidable, resection of major structures such as the SVC and phrenic nerve is likely futile and not worth the associated morbidity.
Tumor marker normalization is usually associated with peripheral fibrosis of the residual mass. With persistently elevated tumor markers, viable tumor is likely present. However, there is no definitive rule as to when a major structure should be resected (2).
Resectable structures
Thymus and lung
There are various resectable structures in the mediastinum encountered when dealing with these tumors. Some require reconstruction and others do not, or cannot, be reconstructed. In general, the entire thymus can be resected and much of the lung as long as the patient’s pulmonary function can tolerate it. Most of these tumors arise in the thymic tissue so en bloc resection of the thymus is often necessary.
Lung resection is often required to obtain negative margins and to provide adequate exposure of the mediastinal structures posterior to the mass. Wedge resection of the peripheral lung with tumor adherence is often all that is necessary to obtain negative margins. Parenchymal sparing resection is strongly preferred. In rare cases with hilar structure invasion, lobectomy or pneumonectomy may be required. However, major pulmonary resection should be avoided whenever possible. Interestingly, the lung can appear deceptively small (like a grapefruit) in these patients when very large tumors compress it. After complete mobilization of the mediastinal portion and with tumor lifted off the mainstem bronchus, the affected lung is inflated with positive pressure ventilation. With the lung re-expanded planes of dissection become clearer between lung and tumor (2).
Pericardium
The posterior extent of mediastinal GCTs is usually the pericardium, and resection of this structure is not always required for negative posterior margins. However, if the posterior margin is in question, en bloc resection of the pericardium is indicated. In general, we reconstruct the pericardium to avoid herniation on the right and constrictive pericarditis on the left. It is also useful in all areas if recurrence occurs, as it protects the underlying heart on reoperation. We usually use a thin prosthesis such as GORE® PRECLUDE® (0.1 mm) and perforate it to prevent a pericardial effusion (2).
Other structures: nerves and great veins
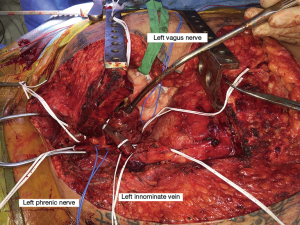
We attempt to preserve the phrenic nerve whenever possible. Preoperative pulmonary function tests should be taken into account when deciding whether to resect the phrenic nerve. One nerve may be sacrificed if the patient’s pulmonary function is adequate and the rest of the tumor is completely resected. To prevent lower lobe atelectasis in the setting of phrenic nerve resection, diaphragmatic plication should be considered. This can be difficult with a midline sternotomy, and if the tumor is more unilateral, a hemi-clamshell is the most useful incision. With regard to the vagus nerves, if tumor is closer to the arch, it is important to consider the location of the recurrent. It should be preserved if possible and the surgeon should be careful with traction. On the right side, tumor is rarely near the right recurrent by the subclavian artery.
When tumor is adherent to the SVC, it can generally be adequately excised by sharp dissection. However, in cases of obvious or suspected SVC invasion on the preoperative CT, ensure inferior large bore access, typically via the femoral vein. SVC resection may be performed as previously described (12). With complete resection of the SVC, sparing of the right phrenic nerve is usually not possible.
When the right or left innominate vein is the only preclusion to complete resection, en bloc resection with the main specimen is recommended. Resection of a single innominate vein may cause transient arm swelling or may have no significant sequelae. However, resection of both innominate veins should generally be accompanied by graft interposition (2).
Postoperative management
Postoperative chemotherapy is indicated when final pathological evaluation demonstrates residual viable tumor. Postpericardiotomy syndrome presenting as fever, pain at the tip of the scapula, pericardial effusion, and diffuse ST segment elevations may occur after resection and treatment with ibuprofen or other nonsteroidal anti-inflammatory drugs (NSAIDs) is usually effective (2).
Equipment preference card
- Favaloro mammary retractor;
- GORE® PRECLUDE® (0.1 mm) patch for pericardial reconstruction;
- Standard thoracotomy instruments;
- Intraoperative nerve monitoring for recurrent laryngeal nerve.
Role of team members
- Anesthesia team:
- Lung isolation: request lung isolation by either blocker or double-lumen tube;
- Intravenous access: lower extremities if innominate vein or SVC may be compromised;
- Nerve monitoring: consider recurrent laryngeal nerve monitoring if tumor extends into the neck or posteriorly.
- Assistants: surgical team members must be comfortable with vascular reconstruction if near the great veins.
Tips & pitfalls
- Consider diaphragmatic plication after phrenic nerve resection;
- GCTs may contain non-germ cell histologies and lead to misdiagnosis on biopsy. These include lymphoma, nephroblastoma, adenocarcinoma, rhabdomyosarcoma, synovial cell sarcoma, and primitive neuroectodermal tumor (PNET);
- A tumor histology of predominately choriocarcinoma is associated with hemorrhagic tendency. Open surgical biopsy, mediastinoscopy, or bronchoscopy should be performed with caution as there is increased risk of significant bleeding;
- Chemotherapy should not be delayed for major surgical interventions whether for biopsy, subtotal, or complete resection;
- Preservation of at least one phrenic nerve should always be ensured;
- Obtain vascular access both superiorly and inferiorly whenever SVC resection is a possibility.
Conclusions
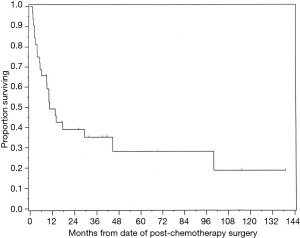
These are aggressive tumors and this patient population has poor long-term survival despite radical surgery (Figure 9). However, multi-modality treatment including surgery portends the best prognosis. Balancing the amount of resection to obtain clear margins versus affecting the quality of life of the patients is the most difficult decision of these surgeries which typically occur in young patients. In a report of 32 patients from one institution (11), histologic analysis of tumors resected following chemotherapy revealed viable tumor in 66%, teratoma in 22%, and necrosis in 12% of specimens. Of those with viable tumor, histologic diagnosis included seminoma, yolk sac carcinoma, choriocarcinoma, embryonal carcinoma, and teratoma with transformation to a non-germ cell malignancy such as sarcoma. The high incidence of residual tumor viability leads us to advocate aggressive surgical technique while keeping in mind that some areas may be involved with fibrosis or residual tumor that is teratoma rather than malignant disease.
Acknowledgments
Special thanks to John Sfakianos, Reza Mehrazin and Rami Tadros for their insight and operative pictures.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Tommaso Claudio Mineo) for the series “Mediastinal Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.06.05). The series “Mediastinal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hazzard C, Kaufman AJ, Flores RM. Mediastinal Tumors. In: Pass HI, Ball D, Scagliotti GV. editors. The IASLC Multidisciplinary Approach to Thoracic Oncology. Aurora, Colorado: IASLC Press, 2014:759-64.
- Flores R. Surgical Management of Primary Mediastinal Germ Cell Tumors. [cited 2018 Apr 7]; Available online: http://ctsnet.org/article/surgical-management-primary-mediastinal-germ-cell-tumors
- Kay PH, Wells FC, Goldstraw P. A multidisciplinary approach to primary nonseminomatous germ cell tumors of the mediastinum. Ann Thorac Surg 1987;44:578-82. [Crossref] [PubMed]
- Nichols CR, Saxman S, Williams SD, et al. Primary mediastinal nonseminomatous germ cell tumors. A modern single institution experience. Cancer 1990;65:1641-6. [Crossref] [PubMed]
- Albany C, Einhorn LH. Extragonadal germ cell tumors: clinical presentation and management. Curr Opin Oncol 2013;25:261-5. [PubMed]
- Singh HK, Silverman JF, Powers CN, et al. Diagnostic pitfalls in fine-needle aspiration biopsy of the mediastinum. Diagn Cytopathol 1997;17:121-6. [Crossref] [PubMed]
- Mazumdar M, Bajorin DF, Bacik J, et al. Predicting outcome to chemotherapy in patients with germ cell tumors: the value of the rate of decline of human chorionic gonadotrophin and alpha-fetoprotein during therapy. J Clin Oncol 2001;19:2534-41. [Crossref] [PubMed]
- Loehrer PJ Sr, Gonin R, Nichols CR, et al. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol 1998;16:2500-4. [Crossref] [PubMed]
- Korst RJ, Burt ME. Cervicothoracic tumors: results of resection by the "hemi-clamshell" approach. J Thorac Cardiovasc Surg 1998;115:286-94; discussion 294-5. [Crossref] [PubMed]
- Bains MS, Ginsberg RJ, Jones WG 2nd, et al. The clamshell incision: an improved approach to bilateral pulmonary and mediastinal tumor. Ann Thorac Surg 1994;58:30-2; discussion 33. [Crossref] [PubMed]
- Vuky J, Bains M, Bacik J, et al. Role of postchemotherapy adjunctive surgery in the management of patients with nonseminoma arising from the mediastinum. J Clin Oncol 2001;19:682-8. [Crossref] [PubMed]
- Venuta F. Surgery of the Superior Vena Cava: Resection and Reconstruction. [cited 2018 Apr 7]. Available online: https://www.ctsnet.org/article/surgery-superior-vena-cava-resection-and-reconstruction
Cite this article as: Hazzard C, Flores R, Nicastri DG. Mediastinal surgery: modern treatment of primary germ cell tumor of the mediastinum. J Vis Surg 2018;4:129.