Surgical minimally invasive techniques for mitral valve repair—cannulation techniques and the Vienna approach
Patient selection and workup
Proper planning with excellent imaging is the key to success in minimally invasive mitral valve surgery. A computed tomography scan with and without contrast agent is performed in every single patient undergoing heart surgery at our center. This allows to detect calcifications of the ascending aorta and to identify soft plaques—therefore serving as a guide to find the best cross-clamping site and to detect the presence of calcifications or tortuosity of the femoral vessels in order to select the best cannulation site. We routinely approach the groin vessels for peripheral cannulation; in case of contraindications for this approach (calcifications, tortuosity) or specific risk for perforation or dissection we perform central cannulation by extending the skin incision with 1–3 cm or plan full sternotomy.
Preoperative preparation
External defibrillating pads are placed prior to draping. The patient is positioned with the right side of the chest elevated to 30 degrees. The anesthesiologist has to ensure a proper muscular relaxation to allow the diaphragm to fall down and to enhance surgical exposure. We do not recommend the placement of a double lumen endotracheal tube—in case of mitral insufficiency with unilateral lung edema the risk of retrograde over-perfusion has to be considered. We routinely perform double venous cannulation. Therefore, sterile washing and draping of the patient is extended towards the right jugular vein, where the anesthesiologist places the guidewire. A TEE probe is placed pre-operatively to support the cannulation maneuvers and to evaluate the mechanisms of mitral valve regurgitation. It may be retracted when the valve is approached in order to enhance the exposure.
Equipment preference card
- Full HD 3D camera and monitor (Einstein Vision Aesculap, Tuttlingen, Germany) or;
- 2D Olympus Evis Exera II CV-180 Camera System (Tokyo, Japan):
- Optics: 5 mm Optics 0°, alternatively 5 mm Optics, 30° or 10 mm Flexible Optics 0°;
- Endoport: 5 or 10 mm port.
- Edwards Soft Tissue retractor medium (Edwards Lifesciences LLC, Irvine, California, USA);
- Minimally-invasive mitral/tricuspid set (Geister ValveGate, Tuttlingen, Germany):
- Geister mini-thoracotomy retractor;
- Aortic clamp: Chitwood (Scanlan, Saint Paul, Minnesota, USA) or Geister clamp;
- Left atrium retractor (blades with holders and introducer);
- 1× Endo-holder for blades (with Nr.11 blade);
- 2× Geister Endo needle-holder;
- 2× Geister Endo-graspers;
- 1× Geister Suture Ruler (Chordae Tendineae measuring device for PTFE sutures);
- 1× Knot-pusher;
- 1× curved Endo-scissors;
- 1× Potts Endo-scissors Potts—(leaflet resection, opening the left atrium);
- Suture-catcher.
Sutures:
- Sutures for open groin cannulation in Seldinger technique:
- 4×5-0 Prolene RB-1/RB-1 (Ethicon, Cincinnati, OH, USA), (purse sutures for arterial and venous cannulation).
- Sutures for percutaneous groin cannulation in Seldinger technique:
- 2× ProGlide systems (Perclose ProGlide Suture mediated closure system (Abott, Illinois, USA)—arterial cannulation;
- Dilators to 24 F, Pigtail catheter, Amplatz-Superstiff wire (venous cannulation).
- Sutures for thorax closure
- Heavy PDS-Chords;
- 5-8×0 Polysorb (Covidien, Medtronic, Minneapolis, MN, USA) middle needle 12×45 cm (pectoralis muscle);
- 4-0 Maxon (Covidien)—(intracutaneous running suture) or;
- 3-0 Ethilon (Ethicon) PS-1 single sutures.
- Other sutures for cannulation, stay sutures, secure sutures:
- 1×3-0 Prolene SH/SH with pledgets (cardioplegia catheter);
- 1×4-0 Prolene SH/SH (atrium stay suture—exposure);
- 4×3-0 Ethibond SH (Ethicon)—(Pericardial stay sutures, 1× towards left, 3× towards right);
- 2×3-0 Prolene SH/SH (left atrium closing suture);
- Optional: 1×4-0 Prolene SH/SH (as adventitia purse suture when the antegrade cardioplegia suture is bleeding);
- Optional: 1×3-0 Prolene SH/SH (atrium retraction).
- Sutures for mitral valve replacement:
- 12-18×2/0 Premichron (B.Braun Surgical S.A., Rubi, Spain) HRC22/HRC22 (SC-Halfcircle 22 mm) with PTFE Pledget (6×3×1.5 mm oval) or;
- 2-18×2-0 Ti-Cron Y-5/Y-5 Cardiopoint (Covidien), (SC-Halfcircle 26 mm) with PTFE Pledget (7×3×1.5 mm sharp tip).
- ϖ Suture material for mitral valve repair:
- 10-16×2/0 Premichron HRC26/HRC26 (SC) Mitral valve ring sutures.
- In case of artificial chords:
- 1-4× Santec Loops ePTFE (Santec, Grosswallstadt, Germany) CV-4/CV-4 with double Pledgets (6×3×1.6 mm), 10–26 mm length—chordae tendineae replacement;
- 2-8× Goretex (GORE-TEX Suture, Goremedical, Flagstaff, Arizona, USA) CV-4 TH-22/TH-22 (small needle) or TH-26/TH-26 (larger needle)—loop suture on cusp.
- Optional when loops are not used:
- 2-8× Goretex CV-4 TH-22/TH-22 (small needle) or TH-26/TH-26 (large needle)—chordae tendineae replacement without loops.
- In case of leaflet’s sutures:
- Cardionyl 4/0/4/0 20 mm needle (Peters Surgical, Bobigny Cedex, France);
- Cardionyl 5/0/5/0 18 mm needle;
- Cardionyl 6/0/6/0 12 mm needle.
- In case of required leaflets adaptation to ring:
- 1-2×5-0 Prolene RB1/RB-1.
- In case of ring plication:
- 1-2×2-0 Ti-Cron Y-5/Y-5 Cardiopoint (SC-Halfcircle 26 mm) with PTFE pledget (7×3×1.5 mm).
- In case of left atrial appendage closure (from inside):
- 1×3-0 Prolene SH/SH.
Cannulation:
- Arterial:
- Chitwood/Glauber clamp: Edwards Femflex II or OptiSite femoral arterial cannula 16, 18, 20 Fr; a Biomedicus arterial cannula 17, 19, 21 Fr may be used for percutaneous cannulation;
- Arterial/Heartport Endoclamp (Cardiovations Port Access Femoral arterial cannula) 21 und 23 Fr/Endoclamp.
- Single venous cannulation (not applied routinely in our center):
- 1st option: Estech RAP (Remote Access Perfusion) Femoral venous cannula 22 Fr [200–100] less than 4.2 l flow or 23/25 Fr [200–150], to 5.2 l flow, with −50 mmHg vacuum to 6.2 l/min. This cannula has to be placed 5 cm in the SVC under TEE view; this allows opening of the right atrium if tricuspid valve surgery is required, as this cannula does not have any holes in the middle. In case of percutaneous cannulation a 23/25 RAP cannula is the best option due to the appropriate stiffness.
- 2nd option: Cardiovations Quickdraw femoral venous cannula 22 Fr less than 4.5 l flow or 23–25 Fr to 5.2 l flow;
- 3rd option: Edwards FemTrak Femoral cannula 24 Fr (Code VFEM024), less than 4.5 l flow; the 28 Fr is too large.
- Double venous cannulation:
When a flow higher than 5.0 liters/min is required or combined procedures as TVr, ASD, atrial myxoma or biatrial Maze are planned
- SVC—1st option: Edwards Femflex II arterial cannula, 16 Fr;
- IVC—1st option: Cardiovations Quickdraw femoral venous cannula, 22 Fr;
- Cardioplegia—DLP Medtronic Dual Lumen Aortic root cannula 31 cm, 11 Fr (11014L);
- Vent—Medtronic DLP Vent 16 F.
Procedure and role of team members
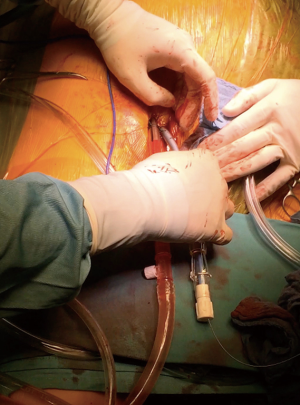
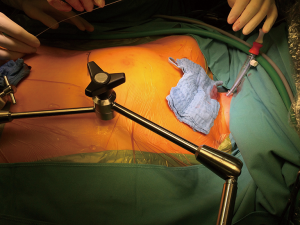
Minimally invasive approaches for the mitral valve with different perfusion strategies have evolved over several years and optimal results can be achieved (1-5). Herein we provide details regarding our minimally invasive mitral valve replacement or repair technique with special emphasis on cannulation strategy. Cannulation for cardiopulmonary bypass is mostly performed through an entirely peripheral approach (Figure 1) at our institution. An additional venous cannula is usually inserted in the right internal jugular vein (Figure 2), especially when a flow greater than 5 L is required or additional procedures like tricuspid valve reconstruction, ASD closure or biatrial MAZE are performed. However, in order to guarantee an optimal venous drainage, two cannulas are routinely used at our institution; in this case, the anesthesiologist places a wire in the internal jugular vein before draping. Thereafter, the femoral cannulation and the thoracotomy are performed simultaneously. Routinely, femoral arterial and venous cannulation is performed either under open view or percutaneous (Figure 1), using two Proglide devices for arterial cannulation. Both cannulas are introduced in Seldinger technique under TEE guidance. In case of direct femoral cannulation, two 5-0 polypropylene RB1 purse sutures for each cannula are used and snugged with tourniquets. The femoral cannulation starts with the venous cannula; the wire is placed in the SVC under TEE control; if this position is difficult to achieve, additional accessories as fluoroscopy and a stiff wire placement through a pigtail catheter are considered. In case of double venous cannulation we choose a Cardiovations Quickdraw 22F femoral cannula, which is positioned with the tip in the IVC and secured with the tourniquets. An Edwards OptiSite arterial cannula (16F, 18F, 20F or 22F) is the 1st option for the arterial cannulation via surgical cutdown. The wire is placed in the descending aorta under TEE control and the vessel is predilated with appropriate dilators. The cannula is placed with the tip in the iliac artery and fixed to the skin with two 2-0 Ethibond stay sutures. In case of percutaneous cannulation, we start with the venous followed by arterial cannulation—the femoral artery being secured with two pre-closing devices.
Thoracotomy
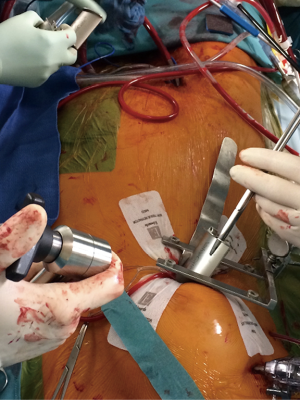
A 6–8 cm inframammary skin incision is performed, starting 1 cm below the nipple in men (1/6 medial and 5/6 towards lateral) and in breast crest in woman to mid-axillary line to allow access in the 4th intercostal space. Before opening the pleura, the ventilation is ceased and the lungs are disconnected with 30 seconds ahead; some surgeons prefer to open the pleura with scissors in order to prevent any injury of the lung. A 1 cm port incision for the Chitwood clamp and vent is performed in the 3rd intercostal space, posterior to the major pectoralis muscle, between the anterior and midaxillary line. The incision can be reduced if the Glauber clamp is used. A 5 mm port incision for the straight camera-optic is performed in the 3rd intercostal space between anterior axillary line and midclavicular line. Alternatively, an angulated camera-optic can be applied in the 4th in intercostal space. The incision is enlarged if a 3D optic system is applied. A 3 mm incision in the 4th or 3rd intercostal space, lateral to the mammary artery is performed for the left atrium retractor. Heparin may be administrated at this time point, if the preparation at the groin is ready. The soft tissue retractor and a ValveGate minithoracotomy retractor are positioned postero-lateral. Behind the inferior blade of the thoracotomy retractor and fixed to it, a wider blade spatula is positioned, which helps to push down the diaphragm and the liver (Figure 3). Alternatively, when a spatula is not used and the right hemidiaphragm is significantly pushed upwards (obesity), a 2-0 polypropylene double armed stitch with two 1×2 cm Teflon patches is placed in its tendinous dome (6), knotted, and brought up to the skin using a needle-hook device through the 6th or 7th intercostal space on the anterior axillary line.
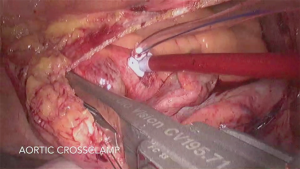
At this time-point the ECC is started and the lungs are disconnected. The pericardium is open at least 2 cm anterior to the right phrenic nerve and four 3-0 Ethibond SH stay sutures are placed through the pericardium (1× towards left, 3× towards right). Now the appropriate level of the thoracotomy should be assessed. Both superior and inferior right pulmonary veins should be in the middle of the operative field. If not, the intercostal space should be changed. The aorta is routinely cross-clamped with a Chitwood or a Glauber clamp (Figures 4,5); when the pulmonary artery cannot be properly separated, it may be clamped as well. CO2 inflation with 3 liters/min is initiated. The interatrial groove is now opened; a SH 4-0 polypropylene stitch is placed through the septum and pulled out on the medial side of the thoracotomy. After X-clamp and cardioplegia administration, the left atrium is opened with the endo-blade. The vent is placed inside the left inferior pulmonary vein to ensure a better view and the left atrium further opened with endo-potts scissors. An appropriate size blade for the left atrium is chosen (Figure 3). The TEE transducer is pulled backwards. To optimize the exposure, a Perier stitch is recommended. Therefore, a 3-0 polypropylene SH suture is placed through P3, 0.5–1 cm away from the mitral ring and parallel to it and then passed out of the left atrium behind the IVC and through the pericardium, afterwards being knotted with a knot-pusher. In this way, an enhanced exposure of the postero-medial commissure is facilitated (this stitch has to be released from tension prior to the water test).

At our institution, a large variety of reconstruction techniques are used as appropriate: ring annuloplasty, triangular or quadrangular resection of the posterior leaflet, the “loop” technique using premeasured polytetrafluoroethylene neochordae (Gore-Tex; WL Gore & Associates Inc., Flagstaff, Ariz), chordal transfer or, commissural plication (Figure 4).
In case of ring annuloplasty or when a mitral valve replacement is required the use of CorKnot© device (LSI Solution, Victor, NY, USA) is extremely beneficial in minimally invasive approaches and used in almost all cases (Figure 4); otherwise a knot-pusher has to be used. After reconstruction/replacement, the vent is placed through the mitral valve into the left ventricle, the retractor is removed and the atriotomy is closed with two 3-0 SH polypropylene running sutures.
For de-airing the heart is filled with blood and compressed. Then the sutures are tied and de-airing is continued through the cardioplegia cannula placed in the aortic root. It is mandatory to place the pacemaker wires before de-clamping the aorta. TEE examination is performed in every single case not only to assess the quality of mitral repair but also to assess the contractility (especially the territory vascularized by the circumflex artery) as well as the integrity of the aortic valve taking into account the anatomical proximity of the mitral annulus and the possible risk of injuring these structures by placing the stitches (8). Every cannulation site is secured with an additional suture. After meticulous hemostasis, the pericardial drain is placed through the port incision used for the clamp and vent; finally the thorax is closed using PDS chordae in figure-of-8 through the 5th rib and at upper margin of the 4th rib, followed by three layers for muscle, subcutaneous tissue and skin.
Post-operative management
Minimally invasive mitral valve cases have proved superiority in terms of postoperative complication in comparison with conventional surgery (9,10). These patients require a shorter postoperative ventilation time, are less prone to perioperative bleedings and could be managed in a fast track regimen with the support and special care from a dedicated anesthesiology and intensive care unit team. The single drain is usually removed on the 1st or 2nd postoperative day and the central venous catheter remains in place 3–4 days in order to facilitate intravenous pain control or administration of other drugs and fluids when required. The patient is discharged on the 6th or 7th postoperative day if no adverse events occur.
Tips, tricks and pitfalls
- Routine use of preoperative CT scan;
- The cannulation is always TEE guided;
- Visualization of the phrenic nerve in order to avoid injury;
- Check for an optimal perfusion during CPP time and complete de-airing of the LV before declamping;
- Place the PM-wires before declamping;
- Post-CPP by means of TEE check for any residual regurgitation, regional contractility (circumflex artery territory) and mobility of the aortic cusps.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Markus Czesla and Javier Gallego) for the series “Mitral Valve Repair Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.03.04). The series “Mitral Valve Repair Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Individual informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chitwood WR, Wixon CL, Elbeery JR, et al. Video-assisted minimally invasive mitral valve surgery. J Thorac Cardiovasc Surg 1997;114:773-80; discussion 780-2. [Crossref] [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Minimally Invasive Port-Access Mitral Valve Surgery. J Thorac Cardiovasc Surg 1998;115:567-74; discussion 574-6. [Crossref] [PubMed]
- Gammie JS, Zhao Y, Peterson ED, et al. Less-Invasive Mitral Valve Operations: Trends and Outcomes From The Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg;90:1401-8, 1410.e1; discussion 1408-10.
- Grossi EA, Loulmet DF, Schwartz CF, et al. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg ;143:S68-S70. [Crossref] [PubMed]
- Santana O, Xydas S, Williams RF, et al. Minimally invasive valve surgery in high-risk patients. J Thorac Dis 2017;9:S614-23. [Crossref] [PubMed]
- Langer NB, Argenziano M. Minimally Invasive Cardiovascular Surgery: Incisions and Approaches. Methodist Debakey Cardiovasc J 2016;12:4-9. [Crossref] [PubMed]
- Coti I, Haberl T, Laufer G, et al. Minimally invasive mitral valve repair. Asvide 2018;5:576. Available online: http://www.asvide.com/article/view/25547
- Czesla M, Götte J, Weimar T, et al. Safeguards and pitfalls in minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:849-52. [PubMed]
- Svensson LG, Atik FA, Cosgrove DM, et al. Minimally invasive versus conventional mitral valve surgery: A propensity-matched comparison. J Thorac Cardiovasc Surg 2010;139:926-32.e1-2.
- Algarni KD, Suri RM, Schaff H. Minimally invasive mitral valve surgery: Does it make a difference? Trends Cardiovasc Med 2015;25:456-65. [Crossref] [PubMed]
Cite this article as: Coti I, Haberl T, Laufer G, Andreas M. Surgical minimally invasive techniques for mitral valve repair—cannulation techniques and the Vienna approach. J Vis Surg 2018;4:126.