
“Getting beyond diameter”: when to replace the aorta?
Introduction
The diameter of the thoracic aorta is a reliable indicator of the severity of aneurysmal degeneration. It is the most important criterion that medical science has identified to estimate the risk of the catastrophic complications, aortic rupture and dissection, and therefore to guide preemptive surgical correction, so as to protect patients from these devastating events (1).
Current guidelines recommend prophylactic surgical intervention at an aortic diameter of 5.5 cm for asymptomatic patients, and between 4.0 and 5.0 cm for Marfan syndrome and other genetically-mediated thoracic aortic aneurysms (TAAs) (2).
The aortic size criterion is extremely valuable, having held up clinically over the years as a dependable guide for prophylactic surgical intervention. However, there is burgeoning evidence (3) that the traditional criteria be revised and also supplemented by other non-size parameters to bring about a more multi-dimensional approach to surgical decision making.
Aortic size criteria: ‘the plot thickens’
Two decades ago, a study based on 230 TAA patients treated at our institution revealed that the incidence of rupture or dissection increased with increasing size of the aneurysm. Specifically, the likelihood of these events increased dramatically at 6 cm for the ascending aorta and 7.2 cm for the descending aorta (4). At that time, we suggested intervening surgically well before these dangerous criteria were met.
Since those early studies, we have been gaining additional clues regarding the appropriate size criterion for intervention.
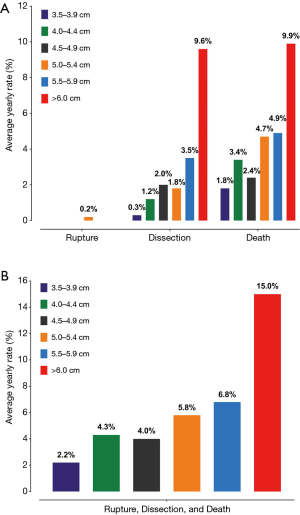
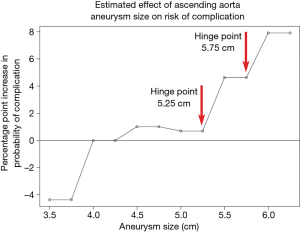
Recently, we reassessed our findings on the natural history of thoracic aortic disease using data from 780 ascending aortic aneurysm patients (5). Again, our analyses showed that the risk of complications increased significantly at an aortic size of 6 cm for ascending aortic aneurysms, with a yearly rate of rupture, dissection and death being 8-fold higher than smaller-sized aneurysms (3.5 to 3.9 cm) (Figure 1). However, the increased granularity of the data (from the larger “n”) allowed us to examine more closely the aortic size range between 5 to 6 cm. We found that in addition to the sharp increase in risk of complications between 5.75 to 6.00 cm, a similarly sharp increase in risk occurred at dimensions between 5.25 to 5.50 cm (Figure 2), earlier than the previous study reported. Stated another way, the increased granularity of data identified two, rather than one, “hinge points” for adverse aortic events.
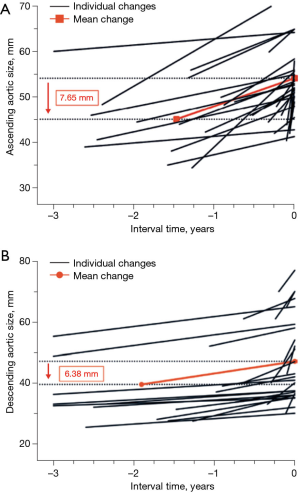
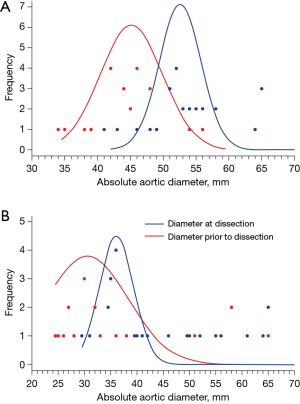
In a 1998 study at our institution, aortic dissection was induced in an animal model by creating an intimal tear and then raising the blood pressure to 300 mmHg using epinephrine (6). Over the course of this study, we noticed that in mere seconds the aorta enlarged at the very moment that the dissection developed. This laboratory observation was validated clinically by a recent study at our institution (7): we showed that the ascending aorta increases in size by approximately 7.65 mm consequent to an acute type A dissection, and the descending aorta by 6.38 mm consequent to an acute type B dissection (Figure 3). These increases in aortic size occur instantaneously at the time of, and as a result of, the acute dissection process itself. Multi-center studies involving acute type A dissection (8) and acute type B dissection patients produced similar results (9). These findings suggest strongly that dissections are occurring at significantly smaller sizes than we initially appreciated, and that current aortic size guidelines overestimate the size threshold for increased risk (Figure 4). This threshold must be located at a much smaller aortic size—representing the true aortic size just prior to the dissection—not the size just after the aortic dissection.
Measurement of the true aortic size has also become problematic with the introduction of the semi-automated, computerized centerline method for assessing aortic dimensions. It is becoming clear that the centerline CT (computerized tomogram) significantly underestimates aortic size as compared to manual measurements (10). Our own comparative studies (unpublished data) confirm this underestimation. Since the aortic size criteria for intervention were all developed using (and are based entirely on) traditional manual estimation of aortic size, the smaller centerline measurements pose a conundrum. If the computerized centerline method is to be widely established, we need to rethink the aortic size criterion. The criterion may need to be placed at a smaller value, reflecting the underestimation of aortic size by the centerline method, compared to the traditional manual measurements from which the criteria were developed.
Taking in to account the issues presented, the aortic size guidelines likely require revision to smaller sizes (3).
Aortic size indexed to biometric data is a more accurate measure of risk than aortic size alone
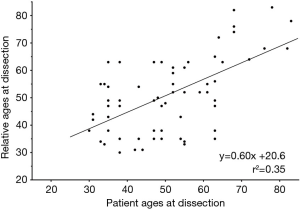
Accounting for biometrics is especially valuable for risk estimation of TAA in patients who are at extremes of body size. It has always seemed perplexing how two patients with great difference in stature could share the same aortic size criterion for intervention. In 2006, we determined that relative aortic size (aortic size indexed to the body surface area of a patient) was a more accurate predictor of the risk of aortic rupture, dissection, or death than aortic size alone (11). This aortic size index (ASI) nomogram (Figure 5) has been widely adopted. Based on the ASI, patients were stratified in to three risk categories and surgical intervention was recommended for patients before they reached an ASI greater than 2.75 cm/m2 in the moderate risk zone.
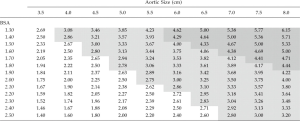
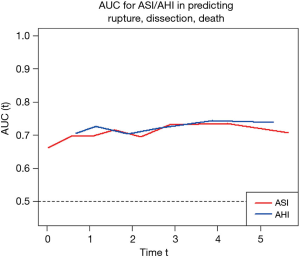
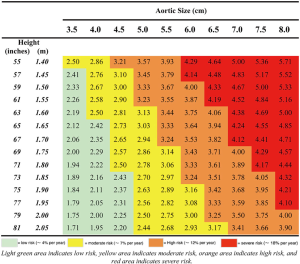
As our database grew since we first introduced the ASI, we were able recently to reevaluate our calculations using a much larger patient population. Analyzing data from 780 exclusively ascending TAA patients, we introduced a new index for risk estimation, the aortic height index (AHI) (5). The earlier ASI calculation, based on body surface area, depended on the weight of a patient; we wondered how the aorta would know (or care) how heavy a patient is. Furthermore, weight can fluctuate over the course of adulthood, so any change in weight would also affect the ASI calculation and hence the risk estimation. Height however, is largely genetically predetermined and remains reliably constant once adulthood is achieved. Accordingly, we evaluated the height-based AHI (aortic size indexed to the height of a patient) and found that the AHI is equal or slightly superior to the ASI in the estimation of adverse aortic outcomes (Figure 6). The AHI also allows us to discard the extra complexity of bringing the body weight and (website or nomogram based) calculation of BSA into the equation. In the AHI nomogram (Figure 7), patients are stratified into four risk categories; we generally recommend aortic surgical repair for patients in the moderate-risk category with an AHI of 2.44 to 3.17 cm/m.
Symptoms should be given precedence over aortic diameter
Symptomatic aneurysms are an exception to the aortic size criteria. Such aneurysms should be resected regardless of the size of the aneurysm (12,13). Only about 5% of patients are symptomatic, but when present, symptoms may indicate threatening aortic pathology and must be given due attention (12). Pain of aortic dissection is characterized as tearing or splitting in nature (14). Before frank dissection occurs, ascending aneurysms can cause a non-exertional retrosternal pain, while pain from the descending aorta can be referred to the back, in the interscapular region (12).
Aortic disease discriminates by gender: female patients require careful surveillance
An unforeseen finding of a 2002 investigation from our institution showed that male sex provided some relative protection in cases of TAAs. We surmised that this difference in risk of complications between genders could be a consequence of a difference in body size relative to aortic size (1). This hypothesis was tested in another study in 2006, in which we found that even with inclusion of BSA in the analysis, the difference in risk withheld (11). Although a lower BSA relative to the aortic size confers a greater risk in all patients, for the female sex the increased risk is not simply due to a difference in body size alone; gender still exerts a deleterious effect in females, above and beyond body size. Some other as yet indeterminate factors contribute (11).
A Canadian study demonstrated that female sex is associated with a greater aneurysm growth rate (15). The growth rate was 1.19±1.15 mm/y in women as compared to 0.59±0.66 mm/y in men (Figure 8). A recent study at our institution corroborates these findings, showing a higher growth rate in female patients (5). Studies have also demonstrated an increase in morbidity and mortality of female patients experiencing acute dissection (16) and undergoing TEVAR (17).
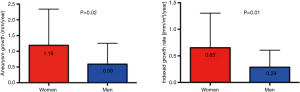
The implication from these studies is that thoracic aortic disease is more aggressive in women, and hence it is beneficial to consider this factor in order to make a timely surgical decision for female patients.
Do anatomical aberrations of the aorta influence disease severity?
Extensive research has been done to evaluate the association of bicuspid aortic valve with presence and progression of thoracic aortic disease. A study at our institution reported a higher relative aortic growth rate for patients with bicuspid aortic valves (18). Of these patients, the ones that had concomitant aortic stenosis had a higher risk of rupture, dissection or death before surgical repair. However, we did not find the expected poorer prognosis for bicuspid patients, nor could our data support intervening at a smaller aortic size in bicuspid patients. The latest expert opinion document (19) no longer recommends a different, lower intervention criterion for bicuspid patients. We used to think of bicuspid disease as a dangerous “Marfan’s light” condition, but this categorization is no longer held to be correct.
Bovine aortic arch has also been associated with TAAs (20). A study at our institution found the aortic growth rate in bovine aortic arch aneurysm patients to be 0.29 cm/year in comparison to 0.09 cm/year for non-bovine aortic arch patients. However, no significant difference in rate of rupture or dissection was found (21).
Although we know that these aortic anatomic conditions are linked with an accelerated aortic growth rate, further research is required to determine if their presence should influence the timing of surgical repair.
A positive family history may indicate a more aggressive disease profile
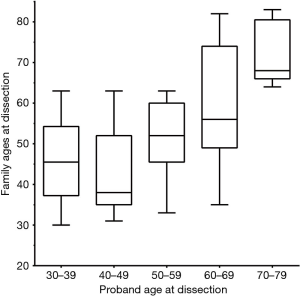
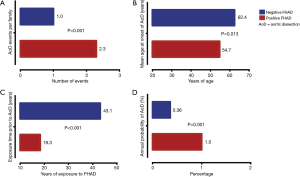
The role of family history in TAA, without frank Marfan’s disease or other known genetic condition, is well established (22,23). The aortic growth rate in familial cases has been shown to be higher than in sporadic cases (0.21 versus 0.16 cm/year) (23). A recent study conducted at our institution demonstrated that the mean age at dissection was significantly less in patients with a positive family history of aortic dissection as compared to those with a negative family history (54.1±15.2 versus 63.1±12.4 years) (24). This study further showed that dissections within the same family have a propensity to occur at a similar age (Figure 9). Greater than 50% of the dissections occurred within a decade of the age at which the proband dissected (Figure 10). Most cogently, we found that, once a first aortic dissection has occurred in any family, other family members face a nearly threefold increased risk of aortic dissection (Figure 11) (25). In light of these results, positive family history should be an important part of the surgical decision-making process. A patient within a decade of the age of dissection of their relative (or even earlier) should receive frequent monitoring of their aorta; early intervention even at moderate aortic size should be strongly considered owing to the increased virulence of this condition once dissection has occurred in a family.
Clues to gain from genetics
Multiple genes linked to TAA and dissection have been identified (26). Heritable thoracic aortic disease can be categorized into syndromic and non-syndromic cases. Syndromic refers to conditions with manifestations in other organs besides the aorta; non-syndromic patients have disease limited to the aorta. Marfan syndrome, which has been associated with hundreds of mutations in the fibrillin-1 gene, accounts for only 5% of all aortic dissection patients (12). Another small percentage is attributed to connective tissue disorders such as Ehlers-Danlos syndrome (mutations in COL3A1) and Loeys-Dietz syndrome (mutations in TGFβR1 or TGFβR2) (27). Mutations in the known heritable thoracic aortic disease genes can currently explain approximately 30% of non-syndromic cases (28).
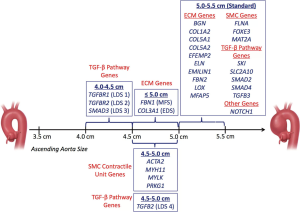
There is evidence to show that specific gene mutations confer a specific increased risk for adverse outcomes, even at small or normal aortic sizes (28). It has been recognized that aortic dissection in Marfan syndrome patients can occur at smaller sizes, therefore we recommend early intervention at 5 cm for the ascending aorta and 6 cm for the descending aorta (14). Figure 12 illustrates our guidelines to assist physicians as to when to intervene with respect to the specific gene mutation a patient harbors.
Genetic testing using whole exome sequencing (WES) is a routine clinical application at our institution (29). With routine genetic testing, patient care can be personalized, and patients carrying particularly aggressive genetic variants can be carefully monitored and triaged to earlier intervention.
The field of genetics is evolving extremely rapidly (with new causative genes discovered yearly) and future developments will provide even greater insight into predicting adverse outcomes based on genetic makeup. Routine widespread WES would help to further refine and enhance the decision for prophylactic surgical extirpation of TAA (29).
A multicenter case-control study confirmed the association of the KIF6 719Arg genetic variant (part of a conventional extended cholesterol screen) with thoracic aortic dissections. Although further work is necessary in this regard, the KIF6 719Arg variant could serve to predict the likelihood of a dissection event (30).
Potential for the application of non-size bioengineering criteria
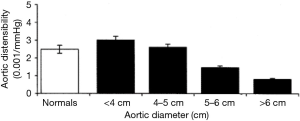
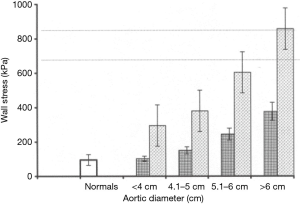
At a diameter of 6 cm the aorta becomes a rigid tube. During systole, instead of being able to dissipate the force generated by myocardial contraction, the critically enlarged aorta stays rigid; all that force of systole is translated in to wall tension (31). In a previous study at our institution, data pertaining to the mechanical properties of the aorta were collected using direct epiaortic echocardiography at the time of aortic surgery in subjects, and at the time of non-aneurysm cardiac surgery in controls (31). Measuring six variables—aortic pressure in systole and diastole, aortic diameter in systole and diastole, and aortic wall thickness in systole and diastole—allowed detailed mechanical profiling of the aorta and confirmed that large aneurysms had lower distensibility (Figure 13) and higher wall stress (Figure 14). We were further able to demonstrate that for an aortic diameter of 6 cm at a blood pressure of 200 mmHg or more (a level achievable during episodes of hypertension as part of everyday life), the wall stress can climb to 857 kPa, very close to the known maximum tensile strength of aneurysmal ascending aortic tissue. Studies are underway to investigate the possibility of determining the mechanical parameters of an aneurysmal aorta using transesophageal echocardiography (or other radiographic means). If successful, this would permit non-invasive out-patient measurements of aortic distensibility and wall stress, providing additional insight for timely surgical repair (32).
The future of the ‘RNA signature’ and biomarkers to supplement aortic size criterion for intervention
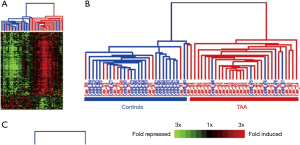
The immune system and inflammatory processes play an important part in the development of TAAs (33). Consequently our research group conducted a study to compare the gene expression patterns in peripheral blood cells of asymptomatic TAA patients with controls (34). The study of 30,000 RNA expression patterns revealed that a 41-gene signature identified TAA patients with an accuracy of over 80% (Figure 15). Work is currently underway to make available a simple blood test which would screen for TAA patients by detecting this “RNA signature”. This will allow early detection of an otherwise silent disease and subsequently careful monitoring with time-appropriate surgical intervention. This RNA signature test measures up and down regulation of aneurysm related RNA’s and informs regarding aneurysmal activity, possibly providing novel clues to predict adverse aortic events.
Various other biomarkers have been studied with hope to diagnose and monitor the progression of TAAs utilizing simple serological tests. Some of these show promise but it still remains to discover a test that can reliably be put in to clinical practice before dissection occurs. D-dimer, a fibrin degradation byproduct, is 99% sensitive in detecting acute aortic dissections (excluding intramural hematomas) but is elevated in many other conditions and so is highly non-specific (35). Of note, D-dimer elevation occurs in consequence to the dissection, eliminating its use as a predictor. Matrix metalloproteinases have also been proposed as a biomarker. In a study by our group we found an increase in MMP-1 and MMP-9, as well as an increase in the ratio of MMP-9 to TIMP-1 (tissue inhibitor of matrix metalloproteinase-1) in TAA patients, signaling an increased proteolysis. Interestingly these findings were more pronounced in aortic dissection patients (36). However, study of MMP’s has not reached a stage of clinical implementation thus far.
PET/CT imaging may provide additional insight
Pioneering FDG-PET/CT imaging studies from Europe (by Sakalihasan, in Liege, and others) have shown increased tracer uptake in aneurysmal tissue (37,38). Increased 18F-fluorodeoxyglucose uptake is associated with increased metabolic activity. The increased metabolic demands of pathologic aortic tissue may allow identification of advanced aneurysms using this technology. FDG-PET/CT imaging could potentially delineate and quantify inflammation of the aneurysmal wall. This has already been found beneficial in distinguishing especially vulnerable aneurysms and guiding surgical intervention. Further investigation of this avenue is needed to bring this criterion into widespread clinical practice.
Safety of aortic surgery in the present era
Finally, the decision to operate on the aorta depends entirely on the safety with which the operation can be performed—with the risk of aneurysmal rupture and dissection being weighed against the inherent risk of the surgical procedure itself. Over the years, aortic surgery has become considerably safer. A 2007 study at our institution involving 506 patients showed that for young patients (<55 years of age) undergoing elective ascending/arch surgery, exemption from permanent complications of surgery (death, paraplegia, stroke) was 98% (39). A recent study by our group investigated 25-year outcomes following composite graft aortic root replacement (40). Operative mortality was found to be 1.9% in elective first-time operations and survival in patients <60 years of age was 92.0%, 90.1%, and 79.8% at 5, 10 and 20 years respectively. Another 2012 study at our institution showed near-equivalent survival compared to the general population in patients undergoing composite aortic root replacement, with only 2% operative mortality for elective operations and a 94.3% and 91.3% freedom from thromboembolism and bleeding at 5 and 10 years, respectively (41).
Conclusions
Aortic diameter is a quantifiable and an extremely powerful predictor of catastrophic aortic events. For many years the aortic size criterion has valuably assisted surgeons with the very important decision of when to replace the diseased aorta. As a predictor of adverse aneurysmal outcomes, aortic diameter indexed to body stature remains relevant and superior to any other criterion, except in rare cases of symptomatic aneurysm presentation (with pain) when surgery is required and size becomes irrelevant.
However, the aortic size criteria need adjustments to accommodate the emerging centerline measurements, which have been shown to underestimate aortic size as compared to manual measurements. There is also mounting evidence that complications may be occurring at smaller aortic sizes than we appreciated. The pre-dissection aorta is much smaller than we recognized and recent analysis points to a sharp increase in risk at a smaller diameter of 5.25 cm. Accordingly, it appears we may need to surgically intervene earlier and at smaller aortic sizes. The technical advancement and increased safety of aortic surgery also make a case for earlier prophylactic intervention.
Nonetheless, to provide meticulous surgical care it is important to consider many other factors that may be individual to each encountered case. A patient’s symptoms, family history, gender, genetics, associated anatomical aberrations of the aorta and, especially genetic profile, should all inform the surgical decision. Further assistance in this decision may be possible in the future with progress in the development of biomarkers, bioengineering, and imaging modalities.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mohamad Bashir and Joseph E. Bavaria) for the series “Innovations in Thoracic Aortic Aneurysm Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.06.04). The series “Innovations in Thoracic Aortic Aneurysm Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27; discussion 27-8. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease. J Am Coll Cardiol 2010;55:e27-129. [Crossref] [PubMed]
- Gryaznov AA, Ziganshin BA, Elefteriades JA. Time to Move to Earlier Intervention for Thoracic Aortic Aneurysm? Structural Heart 2018;2:10-22. [Crossref]
- Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg 1997;113:476-91; discussion 489-91. [Crossref] [PubMed]
- Zafar MA, Li Y, Rizzo JA, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg 2018;155:1938-50. [Crossref] [PubMed]
- Morales DL, Quin JA, Braxton JH, et al. Experimental confirmation of effectiveness of fenestration in acute aortic dissection. Ann Thorac Surg 1998;66:1679-83. [Crossref] [PubMed]
- Mansour AM, Peterss S, Zafar MA, et al. Prevention of Aortic Dissection Suggests a Diameter Shift to a Lower Aortic Size Threshold for Intervention. Cardiology 2018;139:139-46. [Crossref] [PubMed]
- Rylski B, Blanke P, Beyersdorf F, et al. How does the ascending aorta geometry change when it dissects? J Am Coll Cardiol 2014;63:1311-9. [Crossref] [PubMed]
- Rylski B, Munoz C, Beyersdorf F, et al. How does descending aorta geometry change when it dissects? Eur J Cardiothorac Surg 2018;53:815-21. [Crossref] [PubMed]
- Rengier F, Weber TF, Giesel FL, et al. Centerline analysis of aortic CT angiographic examinations: benefits and limitations. AJR Am J Roentgenol 2009;192:W255-63 [Crossref] [PubMed]
- Davies RR, Gallo A, Coady MA, et al. Novel Measurement of Relative Aortic Size Predicts Rupture of Thoracic Aortic Aneurysms. Ann Thorac Surg 2006;81:169-77. [Crossref] [PubMed]
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010;55:841-57. [Crossref] [PubMed]
- Elefteriades JA, Tranquilli M, Darr U, et al. Symptoms plus family history trump size in thoracic aortic aneurysm. Ann Thorac Surg 2005;80:1098-100. [Crossref] [PubMed]
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy's playbook. Curr Probl Cardiol 2008;33:203-77. [Crossref] [PubMed]
- Cheung K, Boodhwani M, Chan KL, et al. Thoracic Aortic Aneurysm Growth: Role of Sex and Aneurysm Etiology. J Am Heart Assoc 2017;6: [Crossref] [PubMed]
- Liang NL, Genovese EA, Al-Khoury GE, et al. Effects of Gender Differences on Short-term Outcomes in Patients with Type B Aortic Dissection. Ann Vasc Surg 2017;38:78-83. [Crossref] [PubMed]
- Deery SE, Shean KE, Wang GJ, et al. Female sex independently predicts mortality after thoracic endovascular aortic repair for intact descending thoracic aortic aneurysms. J Vasc Surg 2017;66:2-8. [Crossref] [PubMed]
- Davies RR, Kaple RK, Mandapati D, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg 2007;83:1338-44. [Crossref] [PubMed]
- Borger MA, Fedak PWM, Stephens EH, et al. AATS Consensus Document on Bicuspid Aortic Valve-Related Aortopathy – Executive Summary. J Thorac Cardiovasc Surg 2018; In press.
- Ziganshin BA, Elefteriades JA. Guilt by association: a paradigm for detection of silent aortic disease. Ann Cardiothorac Surg 2016;5:174-87. [Crossref] [PubMed]
- Hornick M, Moomiaie R, Mojibian H, et al. 'Bovine' aortic arch - a marker for thoracic aortic disease. Cardiology 2012;123:116-24. [Crossref] [PubMed]
- Milewicz DM, Chen H, Park ES, et al. Reduced penetrance and variable expressivity of familial thoracic aortic aneurysms/dissections. Am J Cardiol 1998;82:474-9. [Crossref] [PubMed]
- Albornoz G, Coady MA, Roberts M, et al. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg 2006;82:1400-5. [Crossref] [PubMed]
- Chou AS, Ma WG, Mok SC, et al. Do Familial Aortic Dissections Tend to Occur at the Same Age? Ann Thorac Surg 2017;103:546-50. [Crossref] [PubMed]
- Ma WG, Chou AS, Mok SCM, et al. Positive family history of aortic dissection dramatically increases dissection risk in family members. Int J Cardiol 2017;240:132-7. [Crossref] [PubMed]
- Brownstein AJ, Ziganshin BA, Kuivaniemi H, et al. Genes Associated with Thoracic Aortic Aneurysm and Dissection: An Update and Clinical Implications. Aorta (Stamford) 2017;5:11-20. [Crossref] [PubMed]
- Pomianowski P, Elefteriades JA. The genetics and genomics of thoracic aortic disease. Ann Cardiothorac Surg 2013;2:271-9. [PubMed]
- Milewicz DM, Regalado E. Heritable Thoracic Aortic Disease Overview. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018.
- Ziganshin BA, Bailey AE, Coons C, et al. Routine Genetic Testing for Thoracic Aortic Aneurysm and Dissection in a Clinical Setting. Ann Thorac Surg 2015;100:1604-11. [Crossref] [PubMed]
- Iakoubova OA, Tong CH, Catanese J, et al. KIF6 719Arg Genetic Variant and Risk for Thoracic Aortic Dissection. Aorta (Stamford) 2016;4:83-90. [Crossref] [PubMed]
- Koullias G, Modak R, Tranquilli M, et al. Mechanical deterioration underlies malignant behavior of aneurysmal human ascending aorta. J Thorac Cardiovasc Surg 2005;130:677-83. [Crossref] [PubMed]
- Chau KH, Elefteriades JA. Natural history of thoracic aortic aneurysms: size matters, plus moving beyond size. Prog Cardiovasc Dis 2013;56:74-80. [Crossref] [PubMed]
- Tang PC, Yakimov AO, Teesdale MA, et al. Transmural inflammation by interferon-gamma-producing T cells correlates with outward vascular remodeling and intimal expansion of ascending thoracic aortic aneurysms. FASEB J 2005;19:1528-30. [Crossref] [PubMed]
- Wang Y, Barbacioru CC, Shiffman D, et al. Gene expression signature in peripheral blood detects thoracic aortic aneurysm. PLoS One 2007;2:e1050 [Crossref] [PubMed]
- Ohlmann P, Faure A, Morel O, et al. Diagnostic and prognostic value of circulating D-Dimers in patients with acute aortic dissection. Crit Care Med 2006;34:1358-64. [Crossref] [PubMed]
- Koullias GJ, Ravichandran P, Korkolis DP, et al. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg 2004;78:2106-10; discussion 2110-1. [Crossref] [PubMed]
- Truijers M, Kurvers HA, Bredie SJ, et al. In vivo imaging of abdominal aortic aneurysms: increased FDG uptake suggests inflammation in the aneurysm wall. J Endovasc Ther 2008;15:462-7. [Crossref] [PubMed]
- Kuehl H, Eggebrecht H, Boes T, et al. Detection of inflammation in patients with acute aortic syndrome: comparison of FDG-PET/CT imaging and serological markers of inflammation. Heart 2008;94:1472-7. [Crossref] [PubMed]
- Achneck HE, Rizzo JA, Tranquilli M, et al. Safety of thoracic aortic surgery in the present era. Ann Thorac Surg 2007;84:1180-5; discussion 1185. [Crossref] [PubMed]
- Mok SC, Ma WG, Mansour A, et al. Twenty-five year outcomes following composite graft aortic root replacement. J Card Surg 2017;32:99-109. [Crossref] [PubMed]
- Zafar MA, Farkas EA, Javier A, et al. Are thromboembolic and bleeding complications a drawback for composite aortic root replacement? Ann Thorac Surg 2012;94:737-43. [Crossref] [PubMed]
Cite this article as: Tanweer M, Zafar MA, Saeyeldin A, Gryaznov AA, Puddifant AJ, Erben Y, Ziganshin BA, Elefteriades JA. “Getting beyond diameter”: when to replace the aorta? J Vis Surg 2018;4:124.


