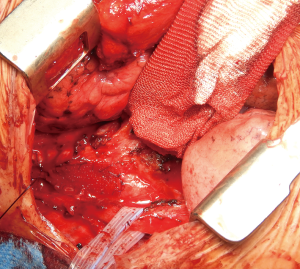
Surgical treatment of acquired benign tracheoesophageal fistulas
Introduction
Acquired fistula between the airway and esophagus is a rare but challenging clinical problem. Granulomatous infection, foreign bodies, and trauma used to be the most common causes of these benign acquired tracheoesophageal fistulas (TEFs) in the past; however, with widespread use of cuffed tubes for ventilation, postintubation fistulae became predominant in the late nineties (1). Recently, though, the etiology of acquired nonmalignant TEFs seems to have changed again; with more and more reports of fistulas arising after esophagectomy and laryngectomy (2-4).
Regardless of the etiology, the life-threatening aspects of this condition are mostly due to the ongoing tracheobronchial contamination, with resulting pulmonary sepsis and interference with nutrition.
Operative closure of TEF is mandatory, because spontaneous closure is exceptional and must not reasonably be expected. Endoscopic intervention with esophageal stents or clips is sometimes attempted (5,6); however, the benefits of such interventions are unproven, while on the other hand they can lead to an extension of the fistulous tract, further complicating definitive management.
The surgical strategy for TEF repair is debated, as several techniques have been proposed. Common general principles include:
- Meticulous preoperative evaluation, including the precise assessment of size and location of the fistula, presence/absence of other associated tracheal or esophageal lesions and determination of patient’s functional status and relevant comorbidities.
- Optimization of patient clinical conditions prior to surgical repair, which implies: weaning of the patient from mechanical ventilation, enteral nutritional support and control of pulmonary sepsis.
- Whenever possible, single stage repair of both tracheal and esophageal defects and interposition of a viable flap to separate the suture lines.
- Early extubation after surgery and avoidance of positive-pressure ventilation.
The goal of this paper is to describe the surgical treatment of TEFs, including all steps of the preoperative management and the different operative techniques.
Initial evaluation
A high index of suspicion is needed in order to promptly detect a TEF. In the mechanically ventilated patient, diagnostic clues include: increased secretions or aspiration of gastrointestinal content from the endotracheal tube, recurrent unexplained pneumonia, sudden abdominal distension, inexplicably low tidal volumes and the phasic inflation and deflation of a plastic nasogastric collection bag synchronously with the respiratory cycle (“breathing bag” sign). In the spontaneously ventilating patient, a suggestive symptom is the sensation of choking while swallowing fluids or solids (Ono’s sign).
Following clinical suspicion, flexible bronchoscopy is often the first diagnostic study. Direct visualization of the fistula can be obtained in mechanically ventilated patients by withdrawing the endotracheal tube or the tracheostomy tube above the suspected fistulous tract.
Esophagoscopy is also routinely performed; although it is less likely to offer a good view, especially of smaller fistulae, it allows the assessment of the esophageal mucosa and the exploration of other pathologic conditions such as ischemia, stenosis or gastroesophageal reflux. Rigid bronchoscopy (Figure 1) offers the best evaluation of the airway, including fistula location and size, its distance relative to the cricoid and carina, the eventual presence and extent of circumferential stenosis. Neck and chest computed tomography can give information about mediastinal and pulmonary infection and damage.

Preoperative preparation
The timing of surgery is a fundamental factor to take into account for operative repair of TEF. In fact, in order to maximize chances of success, aspiration and pulmonary infection need to be under control, nutrition satisfactory, and continued respiratory support no longer required.
To control aspiration, in patients with a tracheostomy, a new tube is positioned with a high-volume, low-pressure cuff inflated below the fistula. Likewise, in mechanically ventilated patients, the endotracheal tube should be positioned precisely under direct fiber-optic guidance with the cuff distal to the lesion but above the carina. The head of patient’s bed is elevated and, if needed, frequent tracheal suctioning is performed.
Oral nutrition is withheld and, if in place, the nasogastric tube is removed in order to relieve the mechanical pressure on the damaged airway. A gastrostomy tube can be useful to drain secretions and gastric content. A jejunostomy tube is positioned to allow post-pyloric enteral feeding.
Due to their prolonged stay in intensive care units, patients become often colonized by multidrug-resistant bacteria at some point during the course of their disease. If infective complications arise, they are treated with specific antibiotic therapy.
When these measures are adopted, the clinical status should quite rapidly improve and weaning from mechanical ventilation is made possible. We believe that this condition prior to surgery is highly advisable, especially if a tracheal resection and anastomosis is required, although some authors have reported satisfactory outcomes of TEF repair in mechanically ventilated patients (2).
Conduct of the procedure
Anesthesia
Close cooperation between the anesthesiologist and surgeon is required in order to maintain the control of the airway during all the course of the operation.
If a tracheostomy is in place, this can be used to initiate anesthesia; however, removal of tracheostomy tube and orotracheal intubation allows for a cleaner surgical field and avoids excessive manipulation of the tube during initial dissection. The endotracheal tube must be positioned under fiber-optic guidance, so as not to let it slip through the fistula and to control correct positioning of the cuff.
If tracheal transection is required, the anesthesiologist will be asked to withdraw the tube and cross-field intubation of the distal trachea will be performed.
For fistulas located in the lower trachea or main bronchi, ventilation is most safely achieved through selective intubation of the non-affected side with a double lumen endotracheal tube or, alternatively, with an extra-long single lumen endotracheal tube.
A nasogastric tube can be positioned, at surgeon’s discretion, to serve as a guide for palpation of the esophagus during dissection. The gastrostomy tube, if present, is put on continuous suction.
Surgical approach
Postintubation fistulae are usually located in the cervical trachea. Thus, in most cases, a collar cervicotomy provides an outstanding exposure of the fistula after tracheal transection. This access can be extended with a partial upper sternotomy or a median sternotomy in order to expose the upper two thirds of the trachea, approximately. When a tracheostomy is present, the skin is incised around the stoma. A left pre-sternocleidomastoid incision can be an alternative approach for TEFs located in the upper trachea, as it gives access to both trachea and esophagus without tracheal transection, when direct tracheal repair is envisaged. Fistulae located at the lower third of the trachea, or bronchoesophageal fistulae, are best approached through a right posterolateral thoracotomy in the 4th intercostal space.
Surgical strategy is dictated not only by the size and location of the fistula, but also by its etiology. Patients with postintubation TEF, in fact, are best treated with tracheal segmental resection and anastomosis, rather than with direct tracheal suture. In these cases, the fistula is a consequence of the mucosal ischemia provoked by the cuff injury, which usually affects the whole circumference of the trachea with frequent association with tracheal stenosis, therefore a direct suturing on a pathologic tissue may cause recurrence of the fistula or it is not feasible. On the other hand, fistulas arising as complication after esophagectomy, due to the presence of extensive necrosis in the neoesophagus or mediastinitis, might at times be most safely dealt with by esophageal diversion than by primary esophageal repair.
Direct closure of the tracheal and esophageal defects
This option is reserved to patients with a small fistula and a normal trachea. Exposure is gained by approaching the fistula from the side by a lateral cervical left pre-sternocleidomastoid incision or by right posterolateral thoracotomy depending on fistula location. Trachea and esophagus are dissected at the location of the fistula, with care taken not to injury the recurrent laryngeal nerve. After identification of the fistulous tract and its division, closure of the membranous tracheal defect is accomplished directly using interrupted sutures of 4/0 polydioxanone (PDS; Ethicon, Inc., Somerville, NJ, USA). The esophageal defect is closed in two layers after debridement of the edges of the fistula. The inner esophageal mucosal layer is closed first with a running suture, inverting the edges of the defect into the lumen, followed by closure of the outer esophageal muscle over the mucosal layer with interrupted 4/0 polydioxanone sutures. In order to avoid close contact of the two suture lines and prevent recurrence, a pedicled flap of strap muscle or of sternocleidomastoid muscle is interposed between the esophagus and the trachea. Alternatively, if a muscle is not available and the site of the fistula is distant enough from the upper esophageal sphincter, separation of the suture lines can be obtained by mobilizing, rotating and fixing the esophagus to the prevertebral fascia.
Tracheal resection and anastomosis with primary esophageal closure
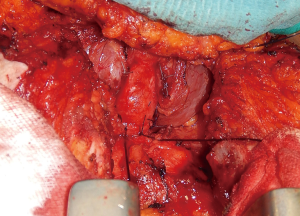
This technique is reserved to those patients with an associated stenosis or circumferential damage to the trachea (Figure 2), or to those patients in which the size of the tracheal defect is excessive for a primary repair without tension. Through an anterior cervical U-shaped incision, the trachea is circumferentially freed only above and below the site of the fistula. The endotracheal tube is withdrawn to just below the level of the vocal cords prior to tracheal transection. A heavy suture placed at the distal end of the tube will facilitate later repositioning. The trachea is then divided and resected below and above the damaged area, and the patient is ventilated by a cross-field endotracheal tube. At that point, the esophageal defect is exposed (Figure 3) and directly closed by suturing in two layers as described above (Figure 4), and a pedicled muscle flap is placed to cover the esophageal suture and to separate it by the tracheal anastomosis (Figure 5). Finally, the tracheal anastomosis is performed following the technique proposed by Grillo et al. (8) and Dartevelle and Macchiarini (9), as follows. A continuous 4/0 polydioxanone suture, running from the left cartilaginous—membranous junction towards the right cartilaginous—membranous junction, is placed, tied, and fixed with two independent polydioxanone sutures whose knots are made outside the lumen (Figure 6). Thereafter, several interrupted stitches of 3/0 polyglactin (Vicryl; Ethicon, Inc.) are placed on the anterior tracheal anastomosis. After all sutures are in place and prior to knotting, the endotracheal tube across the field is removed and the orotracheal tube is advanced into the distal trachea. The anesthesiologist is then asked to put the patient head in slight flexion and the two tracheal edges are gently approached. Sutures are knotted starting from the two lateral aspects of the trachea while anterior sutures are knotted last (Figure 7), and a water leak test is then performed. A sentinel drain is positioned close to the anastomosis and another one into the subplatysmal plane.
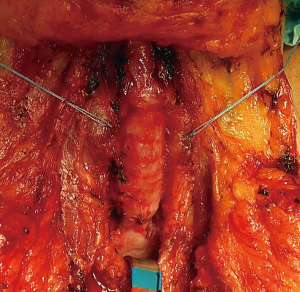
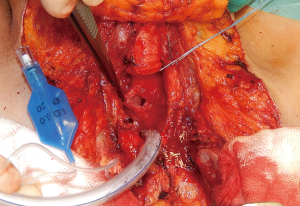
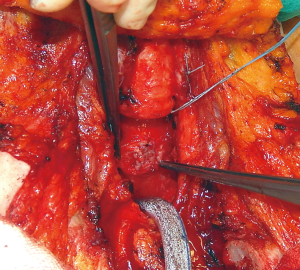
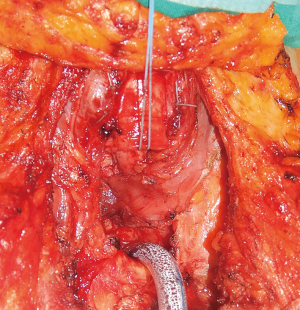

A protective distal tracheostomy is performed in selected cases, as follows: (I) in cases of extensive tracheal resection, in order to decompress the laryngotracheal axes; or (II) when the patient is judged to be at high risk of postoperative mechanical ventilation, owing to poor neurological or respiratory status.
Repair of large airway defects
In some cases, the length of the posterior defect in the membranous wall contraindicates both the direct closure, owing to the risk of dehiscence or stenosis, and the tracheal resection and anastomosis, because the amount of trachea that required resection would be excessive and unsafe (Figure 8). Different alternative techniques can therefore be employed:
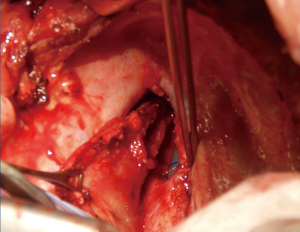
- Adjacent esophageal wall can be used to patch the tracheal defect; either by borrowing a part of its anterolateral aspects (10), or by excluding an entire esophageal segment. This latter procedure, however, requires esophageal diversion and later reconstruction (11).
- A muscle flap can be rotated and sutured over the tracheal defect, as described by several independent groups (4,12). In this case the muscle flap serves both as a separation between the trachea and esophagus and as a patch for tracheal membranous wall. The choice of the muscle flap depends mainly on fistula location. Most useful flaps include intercostal, latissimus dorsi, serratus anterior or pectoralis major muscle flap. The flap has to be appropriately sized to allow a tension-free suture and well-vascularized. If an intercostal muscle flap is used, the parietal pleural surface is preserved and put intraluminally. Suture is performed by the use of resorbable material such as polyglactin or polydioxanone. Complications include necrosis of the flap, with potential for suture dehiscence and TEF recurrence, and excessive protrusion of the muscle into the tracheal lumen, causing dynamic airway obstruction.
- Synthetic bio-absorbable patch use is a useful tool for reconstruction of airway defects, as previously reported by our group (13). Main advantages are constituted by their rigid structure, which can be used as a support for the interposition of a muscle flap; and their synthetic scaffold, which serves as a matrix for epithelial colonization and creation of a neomucosa. The patch can be easily trimmed to fit the posterior membranous defect and suture must be performed with a slight tension to avoid bulging (Figures 9-11Figure 12). According to our previous findings, complete healing with a normal mucosa and a healthy tracheal wall are visible by bronchoscopy after three to six months from the procedure (13).
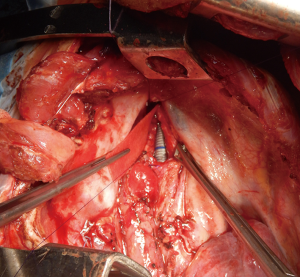 Figure 9 A bioabsorbable patch of synthetic material (Gore Bio-A tissue reinforcement) is being sutured over the defect in the posterior membranous wall of the trachea.
Figure 9 A bioabsorbable patch of synthetic material (Gore Bio-A tissue reinforcement) is being sutured over the defect in the posterior membranous wall of the trachea. Figure 12 Endoscopic view few days after TEF surgical repair, demonstrating the large membranous tracheal wall defect covered by using the bioabsorbable patch (14). TEF, tracheoesophageal fistula. Available online: http://www.asvide.com/article/view/25534
Figure 12 Endoscopic view few days after TEF surgical repair, demonstrating the large membranous tracheal wall defect covered by using the bioabsorbable patch (14). TEF, tracheoesophageal fistula. Available online: http://www.asvide.com/article/view/25534 - In the most complex cases of large fistulas associated with extensive circumferential tracheal damage, a large segmental tracheal resection might be required. While all of the aforementioned techniques can be used for closure of the esophageal and the posterior membranous defects, end to end tracheal anastomosis will not be possible due to the excessive length of the resected trachea. The resulting wide anterolateral tracheal defect is best managed by fashioning of a new tracheostomy, whose orifice is formed by suturing the two tracheal ends and the peritracheal tissue to the skin. In the absence of glottic pathology, positioning of a T-tube is suggested as soon as possible, due to a reduced trauma on the posterior membranous wall, possibility of voice restoration and resuming of oral intake, and less chance of displacement than a tracheostomy tube.
Esophageal diversion
Esophageal diversion is almost never required in the setting of postintubation TEFs. In fact, since the lesion is provoked by the pressure of the endotracheal cuff on the airway, the extent of damage in the tracheal side is normally greater than on the esophageal side, where a two-layered primary suture is always possible. For fistulas arising after esophagectomy, on the contrary, several factors may contraindicate a primary neoesophageal repair, including the presence of mediastinitis due to leakage of the cervical anastomosis and ischemia of the neoesophagus. Furthermore, these patients have often undergone chemo- or radiotherapy, which can lead to diffuse fibrosis and impaired tissue healing. Under these circumstances, esophageal diversion is the safest approach. After division of the esophagus above and below the fistulous tract, which is excised with the involved esophageal segment, a left cervical esophagostomy is performed for drainage of saliva, and a jejunostomy, if not already in place, is made to provide enteral feeding. At a later stage, esophageal reconstruction can be attempted by transposition of the stomach or of a colonic segment, preferably through the substernal route.
Role of team members
Surgical team should be composed of at least 2 surgeons, 1 anesthesiologist, 1 scrub nurse and 1 operating room nurse. The whole team should be aware of the procedure being performed, including the proposed management of the airway, the planned reconstructive interventions and possible backup plans. The operating surgeons must be proficient in airway resection and reconstruction procedures as well as in esophageal surgery. Alternatively, a digestive surgeon needs to be at disposal in case of complex procedures on the esophagus.
The anesthesiologist needs to be familiar as well with airway surgery and rigid bronchoscopy procedures. This includes the use of intermittent apnea, cross-field intubation and jet ventilation. Moreover, the anesthesiologist, or an additional operator, must be capable of performing flexible bronchoscopy, which is necessary at different stages during the procedure; namely intubation, intraoperative fistula localization, control of airway anastomosis and of the final positioning of a T-tube or tracheostomy tube.
Postoperative management
Extubation is performed as soon as possible and positive pressure ventilation is avoided. In patients undergoing procedures of tracheal resection and anastomosis, flexible bronchoscopies are done at postoperative day 1 and day 7 to control the suture line. Additional bronchoscopies are done if clinically indicated. Before resuming oral diet, all patients undergo contrast radiography to confirm healing of the esophagus, while laryngoscopy is performed to assess vocal cords’ and swallowing function. Physiotherapy is promptly initiated in all patients with postoperative dysphagia or vocal cord dysfunction after evaluation by an otolaryngologist. If not already initiated prior to the intervention, broad-spectrum prophylactic antibiotic therapy is administered in the postoperative period.
Patients in whom an airway appliance is left in place or those undergoing esophageal diversion are followed-up regularly, and definitive surgical correction is performed whenever possible.
Conclusions
Surgical management of acquired benign TEFs is complex. A thorough preoperative evaluation is needed in order to choose the best treatment strategy as well as the proper timing for the operation. The procedure of choice, whenever possible, consists in a single stage repair of both tracheal and esophageal defects and interposition of a tissue flap to separate the suture lines. A great degree of individualization is fundamental when dealing with complex fistulas, whose operative management may range from procedures of tracheal resection and anastomosis, muscle-flap or synthetic patch positioning for bridging of airway defects, handling of large tracheostomies through T-tubes or tracheostomy tubes, and esophageal diversion.
The care of patients with TEFs is demanding, and it is best offered by Centers with a dedicated multidisciplinary team including surgeons, anesthesiologists, bronchoscopists, otolaryngologists, infectious disease specialists; as well as intensive care physiotherapists, speech therapists, and dieticians.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the the editorial office, Journal of Visualized Surgery for the series “Tracheal Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.06.07). The series “Tracheal Surgery” was commissioned by the editorial office without any funding or sponsorship. FR served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Visualized Surgery from Jul 2017 to Jun 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thomas AN. The diagnosis and treatment of tracheoesophageal fistula caused by cuffed tracheal tubes. J Thorac Cardiovasc Surg 1973;65:612-9. [PubMed]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8. [Crossref] [PubMed]
- Muniappan A, Wain JC, Wright CD, et al. Surgical treatment of nonmalignant tracheoesophageal fistula: a thirty-five year experience. Ann Thorac Surg 2013;95:1141-6. [Crossref] [PubMed]
- Rosskopfova P, Perentes JY, Schäfer M, et al. Repair of challenging non-malignant tracheo- or broncho-oesophageal fistulas by extrathoracic muscle flaps. Eur J Cardiothorac Surg 2017;51:844-51. [PubMed]
- Dogan UB, Akin MS, Yalaki S. Endoscopic closure of tracheoesophageal fistulas with the over-the-scope clip system. J Coll Physicians Surg Pak 2014;24:S193-5. [PubMed]
- Eleftheriadis E, Kotzampassi K. Temporary stenting of acquired benign tracheoesophageal fistulas in critically ill ventilated patients. Surg Endosc 2005;19:811-5. [Crossref] [PubMed]
- Marulli G, Mammana M, Natale G, et al. Endoscopic view of a TEF located in the upper trachea with a slight circumferential stenosis. Asvide 2018;5:574. Available online: http://www.asvide.com/article/view/25533
- Grillo HC, Moncure AC, McEnany MT. Repair of inflammatory tracheoesophageal fistula. Ann Thorac Surg 1976;22:112-9. [Crossref] [PubMed]
- Dartevelle P, Macchiarini P. Management of acquired tracheoesophageal fistula. Chest Surg Clin N Am 1996;6:819-36. [PubMed]
- Mathisen DJ, Grillo HC, Wain JC, et al. Management of acquired nonmalignant tracheoesophageal fistula. Ann Thorac Surg 1991;52:759-65. [Crossref] [PubMed]
- Bartlett RH. A procedure for management of acquired tracheoesophageal fistula in ventilator patients. J Thorac Cardiovasc Surg 1976;71:89-95. [PubMed]
- Marulli G, Loizzi M, Cardillo G, et al. Early and late outcome after surgical treatment of acquired non-malignant tracheooesophageal fistulae. Eur J Cardiothorac Surg 2013;43:e155-61. [Crossref] [PubMed]
- Battistella L, Marulli G, Comacchio GM, et al. Successful treatment of a recurrent wide tracheoesophageal fistula with a bioabsorbable patch. Ann Thorac Surg 2016;101:e173-5. [Crossref] [PubMed]
- Marulli G, Mammana M, Natale G, et al. Endoscopic view few days after TEF surgical repair, demonstrating the large membranous tracheal wall defect covered by using the bioabsorbable patch. Asvide 2018;5:575. Available online: http://www.asvide.com/article/view/25534
Cite this article as: Marulli G, Mammana M, Natale G, Rea F. Surgical treatment of acquired benign tracheoesophageal fistulas. J Vis Surg 2018;4:123.