
Subxiphoid robotic thymectomy for myasthenia gravis
Introduction
Thoracoscopic surgery is performed while looking at a monitor, which presents limitations because the surgical visual field is two-dimensional (2D); furthermore, this surgery is performed using long instruments. As a result, unnatural surgical manipulations are occasionally required. Robot-assisted surgical systems have been developed for addressing these shortcomings. One of these surgery-assisting robots, the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA), includes robotic arms with multiple articulations and displays clear three-dimensional (3D) images. This robotic system provides a 3D visual field and has articulated forceps that function in a manner similar to the human wrist, which enables natural dissection, resection, and suturing manipulations. In the present paper, the history and current state of robot-assisted thymectomy for myasthenia gravis (MG) are presented, and the surgical procedure for subxiphoid robotic thymectomy for MG is described.
History and current state of robot-assisted thymectomy
Thoracoscopic thymectomy for MG can be performed using three approaches. These include transcervical thymectomy performed via a cervical incisional wound, a lateral thoracic approach performed via a lateral intercostal space, and a subxiphoid approach. In the transcervical thymectomy reported by Cooper et al. in 1988 (1), there is no intercostal nerve damage because it does not pass through the ribs. This has the advantage of minimizing pain; however, this approach is not widely used because of poor operability and difficulty in ensuring the visual field via the neck. Presently, the most frequently used approach in thoracoscopic thymectomy for MG is a lateral thoracic approach via a lateral intercostal space. In 1993, Sugarbaker et al. reported that this approach is advantageous because of excellent esthetics as the incision is made into the lateral chest; however, the disadvantages of this approach are that it always causes intercostal nerve damage, and as shortcomings of surgical operability, it is challenging to ensure the visual field of the neck portion of the thymus and to identify the location of the contralateral phrenic nerve (2,3). In 1999, Kido et al. reported thymectomy using a subxiphoid approach (4). This approach results in minimal pain as it does not pass through the ribs and has an excellent esthetic outcome. However, as with transcervical thymectomy, the subxiphoid approach is not widely used due to poor operability and difficulty ensuring the visual field. In 2012, we reported single-port thymectomy using CO2 insufflation (5). This method, with a greatly improved visual field by CO2 insufflation, is presently the most minimally invasive surgical approach because it does not pass through the ribs; however, it has limited operability. Because all surgical operations for single-port procedures are performed via a single incisional wound, instruments and advanced surgical skills specific to single-port procedures are required. Furthermore, the use of this approach is challenging for complex procedures that require suture operations.
For thoracic surgery using a robot, thymic tumor resection was first reported by Yoshino et al. in 2001 (6), and the first robot-assisted lung cancer surgery was reported by Melfi et al. in 2002 (7). Thereafter, numerous robot-assisted thoracic surgeries have been reported. The existence of robotic articulations that move in the same manner as human articulations facilitates surgical operations, even in the narrow anterior mediastinum. Robot-assisted thymectomy for MG was first reported by Ashton et al. in 2003 (8). Subsequent reports have described positive outcomes of robot-assisted thymectomy for MG (9,10). All these robot-assisted thymectomies had been performed using a lateral thoracic approach; however, in 2015 we reported the subxiphoid approach for robot-assisted thymectomy (11). In thymectomy using the subxiphoid approach, a camera is inserted from the body midline, making it easier to ensure the visual field of the neck and to identify the bilateral phrenic nerves.
The usefulness of robot-assisted surgery in thymectomy for MG
When thymectomy is performed taking a thoracoscopic approach, problems can arise such as difficulty ensuring the space of the anterior mediastinum for performing surgical operations and poor operability in such a narrow space. The anterior mediastinum is the region surrounded by the sternum, heart, and both lungs. In thoracoscopic surgery without sternotomy, a method must be devised for creating a space in the anterior mediastinum. Various methods of lifting the sternum for creating a space in the anterior mediastinum have been reported (4). In 2012, we reported a method of CO2 insufflation in the mediastinum (5). The insufflation of CO2 causes displacement of both lungs and the heart, thereby greatly improving the visual field in the anterior mediastinum. Another technical problem is poor surgical operability using rigid instruments without articulation in the narrow space of the anterior mediastinum. The greatest advantages of surgery performed with the assistance of the da Vinci robot include the precision of the surgical procedure and high operability using multi-articulated instruments. The presence of multiple articulations in a narrow space enables dissections to be performed in a natural manner. This is a major advantage compared with operations using straight instruments in normal video-assisted thoracoscopic surgery (VATS). Therefore, robot-assisted surgery is well-suited for thymectomy, which must be performed in a narrow space.
Approaches used in robot-assisted thymectomy for MG
In 2016, Wolfe et al. reported the usefulness of thymectomy for MG using a median sternotomy approach in a multicenter randomized trial (12). The thymus in patients with MG often develops a germinal center-like structure, and it is believed that the resection of the thymus, including the germinal center, will alleviate the symptoms of MG. In 1981, Masaoka et al. reported that extended thymectomy with extensive resection of the thymus together with the fatty tissue anterior to the phrenic nerve is more effective than simple thymectomy (13), and numerous institutions presently employ this procedure. It has also been reported that the fatty tissue should be extensively resected in addition to extended thymectomy (14). Moreover, when performing thymectomy for MG, sufficient resection of the fatty tissue of the neck and fatty tissue anterior to the bilateral phrenic nerves is necessary for the best outcomes.
Robot-assisted thymectomy is performed at most institutions using a lateral thoracic approach (Figure 1). Most instances of thoracoscopic thymectomy performed by human hands are done so using a lateral thoracic approach because it is a familiar procedure, and respiratory surgeons may be unfamiliar with the subxiphoid approach. However, when using a lateral thoracic approach from one side, it is difficult to identify the location of the contralateral phrenic nerve, and it is impossible to identify the caudal side of the contralateral phrenic nerve. Furthermore, to adequately employ the robot system, the target, i.e., the thymus, should be between the left and right arms; however, in a lateral thoracic approach, the neck part of the thymus is not located between the left and right arms. By contrast, in the subxiphoid approach (Figure 2), a camera is inserted through the subxiphoid incision in the midline of the body (Figure 2), and the same surgical visual field can be obtained as with median sternotomy, with the neck area and location of the bilateral phrenic nerves easily identified. In this approach, the left and right robot arms are inserted via the sixth intercostal spaces of the bilateral precordium, which enable maximum robot performance because the entire thymus is located between the left and right arms. Moreover, with the da Vinci Xi surgical system, the camera/endoscope can be inserted through any port. This means that in the visual field of the camera inserted via a subxiphoid incisional wound, the entire length of the normally concealed left phrenic nerve near the left diaphragm can be observed (Figure 3). Therefore, subxiphoid robotic thymectomy enables complete resection of the fatty tissue anterior to the phrenic nerve.
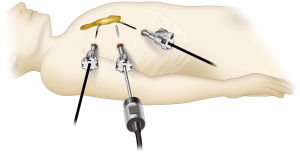
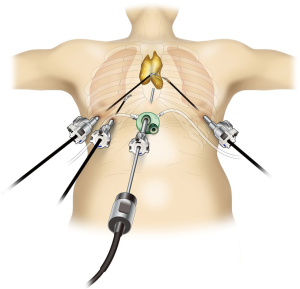

Indication for subxiphoid robotic thymectomy
Because of the higher level of difficulty involved, robot-assisted surgery is more effective than normal thoracoscopic surgery. Accordingly, for patients with non-invasive tumor of the anterior mediastinum and MG patients without a concurrent tumor, subxiphoid single-port thymectomy is indicated because it is minimally invasive. Subxiphoid robotic thymectomy is indicated for patients with tumor invasion of the pericardium requiring pericardial patch closure, and for patients with a tumor in the neck or touching the innominate vein who require innominate vein taping before and after tumor resection. Regarding tumor size, a larger tumor can be more easily removed through a subxiphoid incisional wound than through a lateral thoracic intercostal space. Emphasis is placed on either the minimal invasiveness of surgery or the operability of surgery, depending on the surgeon. For example, in surgery for MG without thymoma, if the surgeon places importance on operability, then robot-assisted surgery rather than single-port surgery should be selected.
Subxiphoid robotic thymectomy
Figure 4 shows a case of taping performed proximal and distal to the tumor, because the tumor touches the innominate vein. In this case, using robot-assisted surgery, a port was inserted into the subxiphoid incisional wound, as well as two ports inserted in the fifth intercostal space of the right lateral chest, and one port inserted in the fifth intercostal space of the left lateral chest.

To begin, a transverse incision of approximately 3 cm is made below the xiphoid process. The linea alba is separated from the xiphoid process for exposing the xiphoid process. There is no requirement for resecting the xiphoid process. To avoid rupturing the peritoneum, a space is made to insert the GelPOINT Mini advanced access platform (Applied Medical, Rancho Santa Margarita, CA, USA), then the GelPOINT Mini with two small ports is vertically connected. The small port on the cranial side is used for the da Vinci camera/endoscope, and the caudal port is used for the assistant. CO2 insufflation is performed in the mediastinum at 8 mmHg. The surgeon detaches the thymus from the posterior aspect of the sternum using the LigaSure Maryland jaw device (Covidien, Mansfield, MA, USA). Bilateral incisions are made into the mediastinal pleura, and the thoracic cavity is exposed bilaterally. While viewing from within the thoracic cavity by thoracoscope, ports for robot-assisted surgery are inserted into two sites in the fifth intercostal space of the right lateral chest, and into one site in the fifth intercostal space of the left lateral chest. The da Vinci Xi surgical system is docked from the left side of the patient and targeted to the neck near the innominate vein. The ports are connected to the da Vinci system, and the surgical procedure is performed with Cadiere forceps as the traction arm attached to the first port on the patient’s right side, fenestrated bipolar soft coagulation forceps attached to the right port medial to the first port, and bipolar Maryland forceps or monopolar forceps with spatula attached to the left lateral chest port of the patient. The resected tumor and thymus are placed in a pouch and removed through the subxiphoid incisional wound. A 20-Fr drain is then inserted through the subxiphoid incisional wound, and the surgery is completed.
Comments
In thymectomy performed within the narrow space of the anterior mediastinum, robotic forceps with articulation that move in the same manner as human wrists are extremely useful for both a lateral thoracic approach and subxiphoid approach. However, a shortcoming of the lateral thoracic approach is that the location of the contralateral phrenic nerve cannot be identified, which is a problem when performing extended thymectomy involving the resection of all fatty tissue anterior to the bilateral phrenic nerves. Furthermore, when the tumor touches the innominate vein, the blood vessel must be safely dissected. Thus, taping with thread is required to be able to clamp the innominate vein at any point proximal and distal to the tumor; however, in a lateral thoracic approach the visual field is disturbed by the tumor and thymus, impeding the view of the tumor and innominate vein on the contralateral side. Consequently, surgery cannot be safely performed because the blood vessel cannot be taped. On the contrary, in robot-assisted thymectomy by a subxiphoid approach, the insertion of a camera through the subxiphoid incisional wound enables observation of the entire innominate vein, and the presence of robotic articulations in the mediastinum enables the surgeon to easily and safely tape the blood vessels. Therefore, robot-assisted subxiphoid thymectomy enables minimally invasive thoracoscopic surgery to be performed on patients who have been unsuitable candidates for thoracoscopic surgery, and indications for thoracoscopic thymectomy have increased. Future improvements in robot-assisted systems may enable surgery that cannot be currently performed by human hands to be completed by robot-assisted surgery.
As for the outcomes of robot-assisted thymectomy compared with those of VATS completed by human hands, no prospective study has compared robot-assisted surgery with VATS. At this time, it is unclear if robot-assisted surgery compared with VATS is beneficial for the patient with MG; however, the advent of robot-assisted thymectomy by the present subxiphoid approach is good news for some patients in that it provides excellent an esthetic outcome. In addition, sternotomy can be avoided, even in patients with tumors of the neck or that are touching the innominate vein. For demonstrating the superiority of expensive robot-assisted surgery over VATS, further prospective multicenter randomized study is required for examining the safety of robot-assisted surgery, evaluating the pain involved, documenting the incidence of complications, and analyzing long-term outcomes. Furthermore, the current system requires the insertion of at least three ports for the left and right hands of the surgeon, as well as a camera. As single-port surgery is currently gaining popularity, it may be necessary to reduce the number of ports in robot-assisted surgery. Robot systems for single-port surgery may serve as an extremely useful tool in subxiphoid thymectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Tommaso Claudio Mineo) for the series “Mediastinal Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.05.24). The series “Mediastinal Surgery” was commissioned by the editorial office without any funding or sponsorship. TS serves as an unpaid editorial board member of Journal of Visualized Surgery from Feb 2018 to Jan 2020. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49:i54-8. [PubMed]
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 2001;122:783-5. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Ashton RC Jr, McGinnis KM, Connery CP, et al. Totally endoscopic robotic thymectomy for myasthenia gravis. Ann Thorac Surg 2003;75:569-71. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Masaoka A, Monden Y. Comparison of the results of transsternal simple, transcervical simple, and extended thymectomy. Ann N Y Acad Sci 1981;377:755-65. [Crossref] [PubMed]
- Jaretzki A 3rd, Penn AS, Younger DS, et al. "Maximal" thymectomy for myasthenia gravis. Results. J Thorac Cardiovasc Surg 1988;95:747-57. [PubMed]
- Suda T. Identification of the location of the left phrenic nerve in subxiphoid robotic thymectomy. Asvide 2018;5:534. Available online: http://www.asvide.com/article/view/25190
- Suda T. Subxiphoid robotic thymectomy. Asvide 2018;5:535. Available online: http://www.asvide.com/article/view/25191
Cite this article as: Suda T. Subxiphoid robotic thymectomy for myasthenia gravis. J Vis Surg 2018;4:120.


