
Novel perspectives in the surgical treatment of posterior mediastinal masses
Introduction
The surgical approach to posterior mediastinal lesions evolves based on the innovative ideas, accumulation of experience, and advances in instrumentation. Video-assisted thoracic surgery (VATS) has already established its position as a reasonable alternative to thoracotomy for the management of posterior mediastinal tumor (1). This great leap forward in surgical technique allowed shorter hospital stay (2-4), minimal postoperative morbidity, and more rapid recovery (2,4-6). VATS itself is also a continuously evolving technique, with single-port VATS becoming increasingly in vogue nowadays. Its safety and benefits for simple or major pulmonary resections were well-demonstrated (7-9). Robot-assisted thoracic surgery (RATS) has also established its role in pulmonary, pleural and anterior mediastinal surgery. In many ways, their applications are also extendable to posterior mediastinal operations.
Single port VATS
The experience of using single-port VATS for major lung resection has been widely reported in recent years (10,11). The reported benefits, compared with conventional 3-port VATS, includes: lower morbidity (9), shorter chest drain duration (9), shorter length of stay (9), no increased mortality (8) or conversion to thoracotomy (8,9), and less blood loss and postoperative pain (7). Furthermore, the technical challenges of instrument fencing and limited visualization have largely been overcome by advances in techniques, scope and instrument design (12-14) (Figure 1).
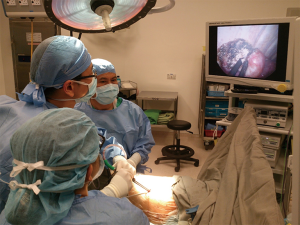
During the course of single port VATS development, much of the reporting and technical refinement, has been focused on pulmonary or anterior mediastinal operations, which harbours the greater proportion of its practice. Therefore, the studies designed for the posterior mediastinal lesions are scarce. Wu et al. reported their experience with single-port VATS in mediastinal lesions in 2015 (15). In the semiprone position they employed, the contralateral hand was placed below the neck, the ipsilateral chest was elevated by 30°, and the ipsilateral arm was flexed and abducted to expose the axillary fossa. The single incision was created in the 4th or 5th intercostal space at the anterior axillary line without the need of rib resection or rib spreading. A 5 or 10 mm 30° thoracoscope was placed at the same incision as the working instruments. Among the 29 mediastinal operations performed, eight were posterior mediastinal lesions (four tumor excision and four cyst excision). The mean wound size was 2.9±0.5 cm, mean operation time was 91±32 minutes, and average blood loss was 35±44 mL. There was no operative mortality. No patients required conversion to three-port VATS or open surgery. The postoperative length of stay was 3.0±0.9 days. No tumour recurrence was noted after a follow-up period of 1–13 months. The population size was further enriched to 40 patients in their succession study in 2016, but the number of posterior mediastinal lesions was not reported (16). Although these case series are limited in their sample size, however, their satisfactory short-term outcomes provide good evidence that approaching the posterior mediastinal lesions via single-port VATS is feasible, safe, and effective. With gradually increasing acceptance of single-port VATS for thoracic surgery, it is expected that larger case series or comparative studies for posterior mediastinal lesions will be reported.
Robotic thoracic surgery
Certain disease factors may render VATS technically difficult and mandate open thoracotomy. Tumors that are large and/or adherent, with invasive or intraspinal growth, or at extreme locations (superior-posterior mediastinum or posterior costodiaphragmatic angle) (17,18) creates difficulties in dissection and manipulation of the target lesion via VATS. Removal of such tumors required very accurate dissections to avoid damaging the surrounding neurovascular structures (19). This may be more easily achievable if aided by the robot systems which offers three-dimensional visualization and greater dexterity. Currently, RATS has already been widely applied to most thoracic operations e.g., major lung resections, pleural operations and thymus resection.
In RATS, patients are usually placed in a lateral decubitus position; further forward body tilting may facilitate the lung and blood to fall away from the operative field for better exposure of the posterior mediastinum. The most commonly used robotic device is the da Vinci system (Intuitive Surgical, Sunnyvale, CA, USA). The camera port is usually positioned in the 7th or 8th intercostal space on the posterior axillary line. The posterior port should ideally be placed in the same intercostal space as the camera port. The anterior port is created in the 5th–6th intercostal space on the anterior axillary line. The fourth robotic arm may be added to aid the retraction or suction (20). Carbon dioxide insufflation may be used to further collapse the lung and flatten the hemidiaphragm (21). To allow optimal dissection and manipulation, the placement of the ports should be modified according to the location of the tumor in the posterior mediastinum. For example, if the targeted lesion is at or infero-posterior to the level of the inferior pulmonary vein, the trocars can be placed anteriorly in the mid or anterior axillary line, and then bring in and dock the robot from the dorsal side of the patient (20).
The only large series of RATS for the posterior mediastinum was reported by Cerfolio et al. in 2012 (20). The procedure was performed in 75 patients with a wide variety of posterior mediastinal pathologies (e.g., tumors, cysts, diaphragmatic hernia, diverticulum). The robotic system was shown to be safe and effective (operative time 95±25 minutes, median blood loss 50 mL, conversion to thoracotomy 1.3%, morbidity 12%, no operative mortality). The robot docking time and the total operative time showed obvious improvement after 10 cases and 25 cases respectively. After Cerfolio’s series, there has been no further significant series or studies to extrapolate the significance of RATS, probably also limited by the relatively smaller incidence of posterior mediastinal lesions and their heterogeneity in pathology. Moreover, the economic and labour intensiveness of setting up a RATS program is high. The monetary and political commitment to purchase the robot, re-design the operating theatre, and the surgeons’ willingness of changing practice are the prerequisites. Additionally, minimally-invasive approach to the posterior mediastinum is intrinsically difficult, regardless of the number of ports and techniques employed, due to the tighter rib spaces posteriorly. To further overcome the technical difficulties and devise the optimal port placements, more efforts are required by the experts in this field to gather and publish their experience and insights. It can also be foreseen that other approaches to resect posterior mediastinal masses may be explored including single port robotic systems, soft robotic systems and even transdiaphragmatic access (10,22-24).
Conclusions
Despite the limited available evidence to mark the advancements in VATS techniques applied to posterior mediastinal lesions, operations via single-port VATS or robot-assisted thoracic surgery are effective, safe and offers satisfactory short-term outcomes. With accumulation of experience and continued technical refinements, minimally-invasive approaches to the posterior mediastinum will gradually establish its role in the treatment of such lesions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Tommaso Claudio Mineo) for the series “Mediastinal Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.05.25). The series “Mediastinal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ng CS, Yim AP. Technical advances in mediastinal surgery: video thoracoscopic approach to posterior mediastinal tumors. Thorac Surg Clin 2010;20:297-309. [Crossref] [PubMed]
- Hazelrigg SR, Boley TM, Krasna MJ, et al. Thoracoscopic resection of posterior neurogenic tumors. Am Surg 1999;65:1129-33. [PubMed]
- Riquet M, Mouroux J, Pons F, et al. Video thoracoscopic excision of thoracic neurogenic tumors. Ann Thorac Surg 1995;60:943-6. [Crossref] [PubMed]
- Bousamra M 2nd, Haasler GB, Patterson GA, et al. A comparative study of thoracoscopic vs open removal of benign neurogenic mediastinal tumors. Chest 1996;109:1461-5. [Crossref] [PubMed]
- Liu HP, Yim AP, Wan J, et al. Thoracoscopic removal of intrathoracic neurogenic tumors: a combined Chinese experience. Ann Surg 2000;232:187-90. [Crossref] [PubMed]
- Han PP, Dickman CA. Thoracoscopic resection of thoracic neurogenic tumors. J Neurosurg 2002;96:304-8. [PubMed]
- Dai F, Meng S, Mei L, et al. Single-port video-assisted thoracic surgery in the treatment of non-small cell lung cancer: a propensity-matched comparative analysis. J Thorac Dis 2016;8:2872-8. [Crossref] [PubMed]
- French DG, Thompson C, Gilbert S. Transition from multiple port to single port video-assisted thoracoscopic anatomic pulmonary resection: early experience and comparison of perioperative outcomes. Ann Cardiothorac Surg 2016;5:92-9. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Yu PS, Capili F, Ng CS. Single port VATS: recent developments in Asia. J Thorac Dis 2016;8:S302-7. [PubMed]
- Ng CS, Kim HK, Wong RH, et al. Single-port video-assisted thoracoscopic major lung resections: experience with 150 consecutive cases. Thorac Cardiovasc Surg 2016;64:348-53. [Crossref] [PubMed]
- Ng CS, Wong RH, Lau RW, et al. Minimizing chest wall trauma in single-port video-assisted thoracic surgery. J Thorac Cardiovasc Surg 2014;147:1095-6. [Crossref] [PubMed]
- Ng CS, Rocco G, Wong RH, et al. Uniportal and single-incision video-assisted thoracic surgery: the state of the art. Interact Cardiovasc Thorac Surg 2014;19:661-6. [Crossref] [PubMed]
- Ng CS, Pickens A, Siegel JM, et al. A novel narrow profile articulating powered vascular stapler provides superior access and haemostasis equivalent to conventional devicesdagger. Eur J Cardiothorac Surg 2016;49:i73-8. [PubMed]
- Wu CF, Gonzalez-Rivas D, Wen CT, et al. Single-port video-assisted thoracoscopic mediastinal tumour resection. Interact Cardiovasc Thorac Surg 2015;21:644-9. [Crossref] [PubMed]
- Wu CY, Heish MJ, Wu CF. Single port VATS mediastinal tumor resection: Taiwan experience. Ann Cardiothorac Surg 2016;5:107-11. [Crossref] [PubMed]
- Al-Mufarrej F, Margolis M, Tempesta B, et al. Novel thoracoscopic approach to difficult posterior mediastinal tumors. Gen Thorac Cardiovasc Surg 2010;58:636-9. [Crossref] [PubMed]
- Podgaetz E, Gharagozloo F, Najam F, et al. A novel robot-assisted technique for excision of a posterior mediastinal thyroid goiter: a combined cervico-mediastinal approach. Innovations (Phila) 2009;4:225-8. [Crossref] [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Video-assisted thoracoscopic extirpation of a posterior mediastinal mass using the da Vinci computer enhanced surgical system. Ann Thorac Surg 2002;74:1235-7. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [Crossref] [PubMed]
- Kocher GJ, Schmid RA, Melfi FM. Robotic lobectomy: tips, pitfalls and troubleshooting. Eur J Cardiothorac Surg 2014;46:e136-8. [Crossref] [PubMed]
- Li Z, Ng CS. Future of uniportal video-assisted thoracoscopic surgery-emerging technology. Ann Cardiothorac Surg 2016;5:127-32. [Crossref] [PubMed]
- Ng CSH, He JX, Rocco G. Innovations and technologies in thoracic surgery. Eur J Cardiothorac Surg 2017;52:203-5. [Crossref] [PubMed]
- Zhao ZR, Li Z, Ng CSH. Trans-diaphragmatic chest surgery: Bringing owls to Athens? J Thorac Cardiovasc Surg 2018;155:1300-1. [Crossref] [PubMed]
Cite this article as: Yu PSY, Ng CSH. Novel perspectives in the surgical treatment of posterior mediastinal masses. J Vis Surg 2018;4:119.


