Ten-year experience with the Perceval S sutureless prosthesis: lessons learned and future perspectives
Introduction
Aortic valve replacement (AVR) for aortic stenosis is one of the most commonly performed cardiac operations (1). For many years, AVR using a surgically implanted prosthesis has been the gold-standard in terms of treatment (2). However, as many as 32% of patients referred for surgical AVR were deemed too high risk for surgery (1). In recent years, transcatheter aortic valve implantation (TAVI) has emerged as a minimally invasive approach to treat these patients. The PARTNER 1 and 2 studies have demonstrated the superiority of TAVI over medical therapy in patients deemed to be inoperable, and the non-inferiority of TAVI in high and intermediate risk patients when compared with surgical AVR (3,4). However, TAVI has been associated with greater rates of paravalvular leaks (PVL), vascular complications and concerns about leaflet thrombosis when compared with conventional AVR (4-8). Moreover, the inability to excise the native valve during TAVI procedures has raised some concerns about the durability of this approach.
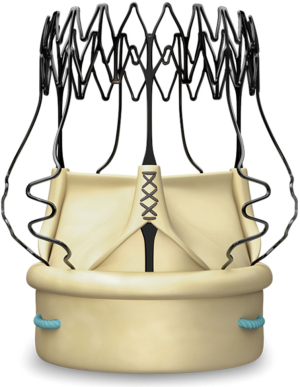
Sutureless valves represent a development in the management of patients undergoing surgical AVR. Their mechanism alleviates the need for suturing and allows for quick deployment of the valve, and thus has the potential to reduce the morbidity associated with long operative time, while still allowing for valve implantation under direct vision. Moreover, the absence of a sewing ring might result in improved hemodynamics when compared with other valves. The Perceval valve is made of bovine pericardium leaflets. It has a self-anchoring, self-expanding nitinol alloy stent that is covered by a thin carbo film coating (Figure 1).
The purpose of this article is to review our 10-year experience with the Perceval prosthesis, focusing on the situations in which we believe it is most useful, some pitfalls, as well as areas of future research.
Clinical data
Multiple non-randomized studies have described the characteristics of the Perceval valve, its strengths and its possible flaws. Short- and mid-term data appear favorable. Published series have reported survival ranging from 86% to 92% at 1 year, 82% to 87% at 2 years and 71% to 85% at 5 years (9). Postoperative hemodynamic characteristics of the Perceval also appear favorable. Mean effective orifice area (EOA) ranges from 1.4±0.4 to 1.6±0.4 cm2, mean gradients range from 10±5 to 14±6 mmHg and mean peak gradients range from 19±8 to 27±11 mmHg in published series (9). In the seminal CAVALIER trial, mean and peak pressure gradients decreased from 45 and 73 mmHg preoperatively to 10 and 19 mmHg at discharge, respectively (10).
Sutureless versus standard AVR
The design of the Perceval allows for quick deployment, thus reducing overall operative time. This is potentially favorable for patients as it might reduce the morbidity associated with prolonged cardiopulmonary bypass (CPB) and cross-clamp time. Multiple studies have demonstrated the adverse effects of prolonged CPB and cross-clamp time (11-13). Ranucci et al. demonstrated a 1.4% increase in severe cardiovascular morbidity for each additional minute of cross-clamp time (11). Various studies have compared CPB and cross-clamp time between isolated surgical AVR with standard stented valves and with the Perceval prosthesis. In a meta-analysis by Powell et al., use of the Perceval prosthesis was associated with a significantly shorter duration of CPB and cross-clamp when compared to standard stented bioprostheses (CPB: 61.4 vs. 84.9 minutes, 95% CI: −27.4 to −18.3, P<0.00001; cross-clamp: 38.6 vs. 63.3 minutes, 95% CI: −24.8 to −16.6, P<0.001) (9). Multiple studies have also reported shorter intensive care unit (ICU) stay for the Perceval valve in isolated AVR and lower incidence of blood transfusion (14-16). However, some publications did show significantly higher rates of permanent pacemaker implantation with the Perceval valve (16-18). Recent studies have identified the presence of a preoperative RBBB, age, chronic renal dysfunction, and QRS duration as predictors of permanent pacemaker implantation following sutureless valve insertion (19,20). To date, no study has shown any statistical differences in terms of mortality when using the Perceval valve versus standard prostheses.
As one would expect, cross-clamp and CPB times are longer in combined cases (i.e., AVR with other concomitant procedure). To date, no trial has specifically addressed the difference in CPB and cross-clamp time between conventional AVR and sutureless AVR with the Perceval prosthesis in a cohort of patients undergoing concomitant procedures. Shrestha et al. performed a cohort study of patients undergoing sutureless with a concomitant procedure. They reported an average CPB and cross-clamp duration of 79±32 and 51±23 minutes, respectively.
Sutureless AVR versus TAVI
In recent years, TAVI has also emerged as a minimally invasive alternative to surgical AVR. Initial studies of TAVI have shown encouraging results in terms of mortality and valve performance for inoperable and high-risk patients (3). However, there remains concern over the rates of stroke, permanent pacemaker implantation, vascular injury and PVL (21,22). Because it is implanted under direct vision, the Perceval valve has the potential to alleviate many of these pitfalls.
Current data on stroke rates does not seem to favor one technology over the other. Rates of perioperative stroke range from 0% to 5% for the Perceval and from 2.5% to 5% for TAVI (14,23-25). A number of studies have shown significantly higher rates of permanent pacemaker implantation with TAVI compared to sutureless AVR with the Perceval (14,26). However, these findings have not been reproduced consistently, and recently published systematic reviews and meta-analyses suggest that there is no significant difference in the rate of permanent pacemaker implantation between these technologies (9,27). These findings should be interpreted in light of the fact that self-expanding TAVI devices are associated with higher rates of conduction disorders than balloon-expandable devices (28).
Few studies have compared rates of vascular complications between TAVI and sutureless AVR. Because sutureless AVR is usually carried out through central cannulation—whereas TAVI relies on peripheral access—it follows that rates of vascular complications will invariably be higher with the use of TAVI. In a study by Biancari et al., no vascular complication was reported in the Perceval group, whereas 11% of patients in the TAVI group had vascular complications (P<0.001) (26). A majority of comparative studies have also shown lower rates of PVL with the use of the Perceval compared to TAVI (14,18,23). The importance of this finding lies in the fact that numerous studies have demonstrated a significant association between any degree of PVL and mortality following TAVI (29). A meta-analysis by Powell et al. showed significantly higher rates of PVL and early mortality in TAVI when compared with Perceval (9). Compared with TAVI, the Perceval has the advantage of allowing complete removal of the diseased valve and decalcification of the aortic annulus, thus reducing the risk of PVL. However, peak and mean transvalvular gradients seem to be lower with TAVI (14,30).
Specific uses
Sutureless valves in minimally invasive approaches
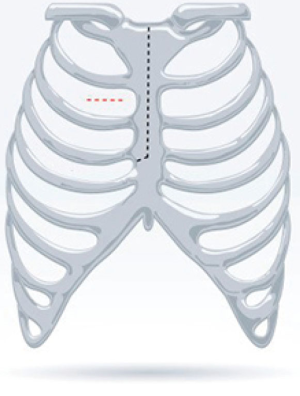
Minimally invasive approaches have various advantages over median sternotomy, including reduced blood loss and shorter ICU length of stay (31). However, minimally invasive AVR can be technically more challenging and is associated with longer CPB and cross-clamp times. Sutureless valves have the potential to overcome these shortcomings. Currently, there are two main techniques that have been described for minimally invasive AVR; the hemi-sternotomy and the right anterior minithoracotomy (Figure 2). The comparative benefits of these approaches—and the potential superiority of one technique over the other—remains a matter of controversy. Miceli et al. reported the largest series of minimally invasive sutureless AVR (164 right anterior thoracotomy and 117 hemi-sternotomy) published to date. They reported a survival of 90% at 1 year in a high-risk cohort of 281 patients. The average CPB and cross-clamp times were 81 and 48 minutes, respectively (32). In a separate study, Miceli et al. reported better results using the right anterior thoracotomy (lower incidence of postoperative atrial fibrillation, shorter ventilation time, shorter ICU stay, and shorter hospital stay) (33). On the other hand, Semsroth et al. found that the right anterior thoracotomy approach was associated with more perioperative complications (higher rates of conversion to sternotomy, longer cross-clamp time, more groin complications) when compared with the hemi-sternotomy approach (34). In a meta-analysis by Powell et al., the rate of conversion from minimally invasive (defined as either hemi-sternotomy or right anterior thoracotomy) to full sternotomy ranged from 0% to 1.4% in published observational studies (9). The authors reported excellent results in terms of mean CPB time (68±18 to 92±27 minutes) and cross-clamp time (34±10 to 59±19 minutes).
Small calcified aortic annulus
Patients with small calcified aortic annuli are good candidates for sutureless AVR with the Perceval for multiple reasons. First, the sutureless design of the Perceval allows easier and faster implantation into the calcified root by alleviating the need for suture passing through a calcified annulus. Second, patients with small aortic roots are at high risk for prosthesis-patient mismatch, especially those with a high body mass index. This is particularly important as prosthesis-patient mismatch has been associated with worse clinical outcomes and decreased survival (35,36). Aortic root enlargement can reduce the risk of prosthesis-patient mismatch. However, this approach is often technically challenging and can result in increased morbidity, especially in heavily calcified roots. Thus, patients with small and calcified aortic annuli may benefit from a sutureless valve since it has been shown to decrease the risk of prosthesis-patient mismatch in this population (37). Moreover, the design of the Perceval valve allows quick and reproducible deployment, even in heavily calcified roots (Figure 1).
Combined procedures
Combined and complex procedures can be associated with prolonged CBP and cross-clamp times, which may result in increased morbidity, especially in elderly and higher risk patients. The use of the Perceval prosthesis for combined cases can decrease overall surgical time. Concomitant mitral valve replacement was initially felt to be a contraindication to the use of the Perceval device, due to concerns over its potential interaction with the mitral prosthesis at the level of the aorto-mitral curtain. However, a number of case series have demonstrated the feasibility and safety of sutureless AVR in this setting (38). In the largest series on combined procedures (i.e., AVR with CABG and/or tricuspid annuloplasty and/or ascending aorta replacement and/or septal myomectomy) published thus far, Shrestha et al. reported mean CPB and cross-clamp times of 79±32 and 51±23 minutes. During a median follow-up of 444 days, 5 valve explanations were performed (4 PVL and 1 aortic root bleeding). The 30-day mortality was 2.1% (39).
Data on early structural deterioration
There have been very few reports of structural deterioration of the Perceval prosthesis. Bouhout et al. (40) reported the first case of early deterioration in a 56-year-old man who presented with symptoms of dyspnea and a transvalvular gradient of 84 mmHg. On intraoperative examination, the prosthesis leaflets were found to be stiffened due to severe calcification. The patient underwent redo-AVR with a mechanical valve and had an uneventful post-operative course. In 2015, Durand et al. (41) described an emergency TAVI in a 78-year-old female patient who presented in cardiogenic shock 3 years after having undergone sutureless AVR with a Perceval valve. The patient had severe aortic insufficiency on transthoracic echocardiography. Unfortunately, a computed tomography scan et transesophageal echocardiography could not be performed due to hemodynamic instability. The suspected mechanism of aortic insufficiency was leaflet tearing. An emergency valve-in-valve procedure using a SAPIEN 3 transcatheter valve was performed and the patient had a quick recovery from her condition. These isolated cases notwithstanding, the Perceval prosthesis seems to have good short and mid-term durability. However, long-term data are lacking.
Special uses and off-label applications
Perceval in the setting of a failing homograft
Dohmen et al. reported the case of a 61-year-old patient who presented with homograft failure after a first redo procedure for prosthetic valve endocarditis and root abscess (42). The patient was initially treated for a type A dissection with aortic root and arch replacement using an elephant trunk. She developed infective endocarditis in the year following her initial surgery and was treated with an aortic homograft. Subsequently, she developed symptoms of dyspnea. Transthoracic echocardiography showed severe aortic stenosis with an orifice area of 0.4 cm2. A 21 mm Perceval valve was positioned in the homograft annulus after careful excision of the homograft leaflets. The patient had an uneventful post-operative course and was discharged on day 6.
Perceval in bicuspid aortic valves
There are currently few data on the use of the Perceval prosthesis in bicuspid valves. Initially, congenital bicuspid valve was considered a contraindication for the use of sutureless AVR, due to concerns over the fact that the aortic annulus in these patients is round rather elliptical. However, Nguyen et al. reported the implantation of the Perceval prosthesis in 25 consecutive patients with bicuspid valves (43). No PVL were observed on pre-discharge transesophageal echocardiography and there was no post-operative valve migration or embolization reported.
Triple-valve surgery
Mazine et al. reported the successful use of the Perceval in 6 patients who underwent triple-valve surgery. For each of these patients, the tricuspid valve was first addressed and annuloplasty was performed. A high transverse aortotomy was subsequently performed to remove the native valve, decalcify and size the annulus. The mitral valve repair or replacement is done through the Sondergaard groove. Finally, the aortic annulus is resized to ensure accuracy of the measurements and the Perceval is deployed. All the patients in the series had a satisfactory postoperative course (44).
Aortic insufficiency
Sutureless valves were initially designed to treat patients with aortic stenosis. Very little literature addresses their use in patients with aortic insufficiency. Gilmanov et al. (45) published a series of 11 patients who underwent AVR for aortic regurgitation. Mean CPB and cross-clamp times were 130±54 and 82±32 minutes, respectively. One patient died in the early post-operative period; the others were successfully discharged. Seven patients were followed up to 1 year. During this period, there was no death, no residual para- or intravalvular leakage and no prosthesis migration/dislocation occurred.
Things we have learned
Since 2011, 577 Perceval devices have been implanted at the Montreal Heart Institute, making it one of the most experienced centers with the use of this technology. Over the years, we have learned to recognize situations in which sutureless AVR with the Perceval prosthesis is most useful, and to recognize and avoid some pitfalls.
Most useful use
Our experience with the Perceval has allowed us to appreciate the rapidity of its deployment and its usefulness in narrow spaces. Thus, we believe that the Perceval is best used in small calcified aortic roots, since it facilitates implantation while still allowing for annular decalcification. Moreover, it alleviates the excess operative risk associated with an annulus enlargement procedure. Furthermore, we find sutureless AVR to be especially useful in minimally invasive procedures, where it facilitates deployment through relatively small incisions. In our experience, favorable results were achieved with both a mini-sternotomy or right anterior thoracotomy approach. Both approaches have been used with low rates of conversion to full sternotomy. Finally, our group has a large experience with the use of the Perceval prosthesis in the setting of double or triple valve surgery, where the use of sutureless technology allows for significant reductions in CPB and cross-clamp times, favorable clinical outcomes, and excellent hemodynamic parameters.
Pitfalls
The rate of permanent pacemaker implantation has been somewhat of a concern with the Perceval. The majority of the literature reports rates of permanent pacemaker implantation between 6% and 9%. However, some studies have reported rates as high as 23% (46). This is a concern, considering the significant morbidity associated with permanent pacemakers (47). Reports have found old age, presence of preoperative rhythm disturbances, height of the membranous septum, presence of a bicuspid aortic valve, and the requirement for concomitant mitral or tricuspid valve procedures to be predictors of post-operative permanent pacemaker implantation (20). Some studies have also suggested oversizing as an additional risk factor (48). During surgery, the risk of post-operative permanent pacemaker implantation can be alleviated by placing the guiding sutures precisely at the nadir of each cusp instead of a few millimeters below, as recommended by the manufacturer. This technical adjustment was first reported by the Toronto group and later confirmed by the Nuremberg group (20,49).
PVL have not been a major concern with the Perceval. The vast majority of published reports show very low rates of moderate or severe PVL (9). Thorough decalcification and sizing are important factors to keep the risk of PVL low.
Adequate sizing is critically important since undersizing can lead to prosthesis migration and oversizing is associated with bleeding complications, arrhythmias and stent invagination. A report by Margaryan et al. indicates that multidetector-row computed tomography might be helpful to determine adequate valve size preoperatively (50).
Areas of future research
The Perceval Sutureless Implant Versus Standard-Aortic Valve Replacement (PERSIST-AVR) study is one of the largest contemporary trials in the field of cardiac surgery (www.ClinicalTrials.gov, Identifier: NCT02673697). This prospective randomized controlled trial, for which enrollment started in 2016, will compare the Perceval prosthesis to other stented biological valves. Its primary endpoint is freedom from major adverse cerebrovascular and cardiac events (i.e., composite of all-cause mortality, myocardial infarction, stroke, and valve re-intervention) at 1 year. The secondary endpoints include operative duration, length of hospital stay, quality of life at 1 month and 1 year, rate of pacemaker implantation and echocardiographic endpoints at various time points (up to 5 years). The study is designed on a non-inferiority model and has a target enrollment of 1,234 patients.
Future research efforts should also attempt to provide high-quality, prospective, randomized data to compare TAVI and sutureless valves in minimally invasive surgery.
Conclusions
Aortic stenosis is a highly prevalent disease, and its incidence is expected to increase further due to an aging population. The development of minimally invasive techniques has allowed surgeons to treat patients with multiples co-morbidities. Among these new technologies, the Perceval sutureless prosthesis offers interesting features. It allows quick and reproducible valve implantation, while still permitting decalcification of the aortic annulus. In our experience, the Perceval valve is most useful in patients with small calcified aortic annuli, concomitant procedure, and during operations carried out through a minimally invasive approach. A higher rate of permanent pacemaker implantation remains the Achilles’ heel of this technology, but recent reports suggest that this risk can be mitigated with careful patient selection and attention to minute technical details. Finally, the Perceval prosthesis has shown good mid-term durability, with very few reports of valve deterioration. In the coming years, prospective, randomized, long-term data comparing the Perceval prosthesis with well-established stented aortic bioprostheses will determine the role of sutureless AVR in the surgical armamentarium. Comparison of minimally invasive sutureless AVR versus TAVI might shed some light on the relative merit of one technology over the other, especially in intermediate-risk patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: D Bouchard is a proctor for LivaNova, London, UK. The other authors have no conflicts of interest to declare.
References
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Walther T, Blumenstein J, van Linden A, et al. Contemporary management of aortic stenosis: surgical aortic valve replacement remains the gold standard. Heart 2012;98:iv23-9. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218-25. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Kodali SK, Williams MR, Smith CR, et al. Two-Year Outcomes after Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Powell R, Pelletier MP, Chu MW, et al. The Perceval Sutureless Aortic Valve: Review of Outcomes, Complications, and Future Direction. Innovations (Phila) 2017;12:155-73. [Crossref] [PubMed]
- Laborde F, Fischlein T, Hakim-Meibodi K, et al. Clinical and haemodynamic outcomes in 658 patients receiving the Perceval sutureless aortic valve: early results from a prospective European multicentre study (the Cavalier Trial). Eur J Cardiothorac Surg 2016;49:978-86. [Crossref] [PubMed]
- Ranucci M, Frigiola A, Menicanti L, et al. Aortic cross-clamp time, new prostheses, and outcome in aortic valve replacement. J Heart Valve Dis 2012;21:732-9. [PubMed]
- Murphy GJ, Angelini GD. Side Effects of Cardiopulmonary Bypass. J Card Surg 2004;19:481-8. [Crossref] [PubMed]
- Al-Sarraf N, Thalib L, Hughes A, et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg 2011;9:104-9. [Crossref] [PubMed]
- Muneretto C, Alfieri O, Cesana BM, et al. A comparison of conventional surgery, transcatheter aortic valve replacement, and sutureless valves in “real-world” patients with aortic stenosis and intermediate- to high-risk profile. J Thorac Cardiovasc Surg 2015;150:1570-7. [Crossref]
- Santarpino G, Pfeiffer S, Concistré G, et al. The Perceval S Aortic Valve Has the Potential of Shortening Surgical Time: Does It Also Result in Improved Outcome? Ann Thorac Surg 2013;96:77-81. [Crossref]
- Dalén M, Biancari F, Rubino AS, et al. Aortic valve replacement through full sternotomy with a stented bioprosthesis versus minimally invasive sternotomy with a sutureless bioprosthesis. Eur J Cardiothorac Surg 2016;49:220-7. [Crossref] [PubMed]
- Forcillo J, Bouchard D, Nguyen A, et al. Perioperative outcomes with sutureless versus stented biological aortic valves in elderly persons. J Thorac Cardiovasc Surg 2016;151:1629-36. [Crossref] [PubMed]
- Muneretto C, Bisleri G, Moggi A, et al. Treating the patients in the ‘grey-zone’ with aortic valve disease: a comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact Cardiovasc Thorac Surg 2015;20:90-5. [Crossref] [PubMed]
- Moran D, DeAsmundis C, Nijs J. 22 Incidence and predictors of permanent pacemaker insertion after perceval sutureless aortic valve implantation – a retrospective single-centre study. Heart 2016;102:A12-3.
- Vogt F, Pfeiffer S, Dell'Aquila AM, et al. Sutureless aortic valve replacement with Perceval bioprosthesis: are there predicting factors for postoperative pacemaker implantation? Interact Cardiovasc Thorac Surg 2016;22:253-8. [Crossref] [PubMed]
- Gargiulo G, Sannino A, Capodanno D, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement: A systematic review and meta-analysis. Ann Intern Med 2016;165:334-44. [Crossref] [PubMed]
- Siontis GC, Praz F, Pilgrim T, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J 2016;37:3503-12. [Crossref] [PubMed]
- D'Onofrio A, Rizzoli G, Messina A, et al. Conventional surgery, sutureless valves, and transapical aortic valve replacement: What is the best option for patients with aortic valve stenosis? A multicenter, propensity-matched analysis. J Thorac Cardiovasc Surg 2013;146:1065-70. [Crossref]
- D'Onofrio A, Salizzoni S, Rubino AS, et al. The rise of new technologies for aortic valve stenosis: A comparison of sutureless and transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2016;152:99-109.e2. [Crossref] [PubMed]
- Santarpino G, Pfeiffer S, Jessl J, et al. Sutureless replacement versus transcatheter valve implantation in aortic valve stenosis: A propensity-matched analysis of 2 strategies in high-risk patients. J Thorac Cardiovasc Surg 2014;147:561-7. [Crossref] [PubMed]
- Biancari F, Barbanti M, Santarpino G, et al. Immediate outcome after sutureless versus transcatheter aortic valve replacement. Heart Vessels 2016;31:427-33. [Crossref] [PubMed]
- Wang N, Tsai YC, Niles N, et al. Transcatheter aortic valve implantation (TAVI) versus sutureless aortic valve replacement (SUAVR) for aortic stenosis: a systematic review and meta-analysis of matched studies. J Thorac Dis 2016;8:3283-93. [Crossref] [PubMed]
- Hoffmann R, Herpertz R, Lotfipour S, et al. Impact of a New Conduction Defect After Transcatheter Aortic Valve Implantation on Left Ventricular Function. JACC Cardiovasc Interv 2012;5:1257-63. [Crossref] [PubMed]
- Kodali S, Hahn R, Williams M, et al. Impact of paravalvular leak following transcatheter aortic valve replacement on one-year mortality: analysis of the combined PARTNER cohorts. Eur Heart J 2013;34:2584. [Crossref]
- D’Onofrio A, Fabozzo A, Gerosa G. Comparison of hemodynamic and clinical outcomes of transcatheter and sutureless aortic bioprostheses: how to make the right choice in intermediate risk patients. Ann Cardiothorac Surg 2017;6:510-5. [Crossref] [PubMed]
- Murtuza B, Pepper JR. Minimal Access Aortic Valve Replacement: Is It Worth It? Ann Thorac Surg 2008;85:1121-31. [Crossref] [PubMed]
- Miceli A, Santarpino G, Pfeiffer S, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: Early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014;148:2838-43. [Crossref] [PubMed]
- Miceli A, Murzi M, Gilmanov D, et al. Minimally invasive aortic valve replacement using right minithoracotomy is associated with better outcomes than ministernotomy. J Thorac Cardiovasc Surg 2014;148:133-7. [Crossref] [PubMed]
- Semsroth S, Matteucci Gothe R, Raith YR, et al. Comparison of Two Minimally Invasive Techniques and Median Sternotomy in Aortic Valve Replacement. Ann Thorac Surg 2017;104:877-83. [Crossref] [PubMed]
- Blais C, Dumesnil JG, Baillot R, et al. Impact of Valve Prosthesis-Patient Mismatch on Short-Term Mortality After Aortic Valve Replacement. Circulation 2003;108:983-8. [Crossref] [PubMed]
- Bleiziffer S, Eichinger WB, Hettich I, et al. Impact of patient-prosthesis mismatch on exercise capacity in patients after bioprosthetic aortic valve replacement. Heart 2008;94:637-41. [Crossref] [PubMed]
- Belluschi I, Moriggia S, Giacomini A, et al. Can Perceval sutureless valve reduce the rate of patient-prosthesis mismatch? Eur J Cardiothorac Surg 2017;51:1093-9. [Crossref] [PubMed]
- Minh TH, Mazine A, Bouhout I, et al. Expanding the indication for sutureless aortic valve replacement to patients with mitral disease. J Thorac Cardiovasc Surg 2014;148:1354-9. [Crossref] [PubMed]
- Shrestha M, Folliguet TA, Pfeiffer S, et al. Aortic Valve Replacement and Concomitant Procedures With the Perceval Valve: Results of European Trials. Ann Thorac Surg 2014;98:1294-300. [Crossref] [PubMed]
- Bouhout I, Noly P-E, Parisi A, et al. First case of Perceval S prosthesis early structural valve deterioration: Not an easy reoperation. J Thorac Cardiovasc Surg 2016;152:e71-3. [Crossref] [PubMed]
- Durand E, Tron C, Eltchaninoff H. Emergency Transcatheter Aortic Valve Implantation for Acute and Early Failure of Sutureless Perceval Aortic Valve. Can J Cardiol 2015;31:1204.e13-5. [Crossref] [PubMed]
- Dohmen PM, Lehmkuhl L, Borger MA, et al. Valve-in-Valve Replacement Using a Sutureless Aortic Valve. Am J Case Rep 2016;17:699-702. [Crossref] [PubMed]
- Nguyen A, Fortin W, Mazine A, et al. Sutureless Aortic Valve Replacement Using the Perceval S Prosthesis: Should Bicuspid Disease be a Contraindication? Can J Cardiol 2014;30:S238. [Crossref]
- Mazine A, Vistarini N, El-Hamamsy I, et al. Sutureless Aortic Valve Replacement in the Setting of Triple-Valve Surgery. Innovations (Phila) 2015;10:291-3. [Crossref] [PubMed]
- Gilmanov DSh, Solinas M, Kallushi E, et al. Sutureless aortic valve replacement for aortic incompetence. J Card Surg 2015;30:391-5. [Crossref] [PubMed]
- Bouhout I, Mazine A, Rivard L, et al. Conduction Disorders After Sutureless Aortic Valve Replacement. Ann Thorac Surg 2017;103:1254-60. [Crossref] [PubMed]
- Cantillon DJ, Exner DV, Badie N, et al. Complications and Health Care Costs Associated With Transvenous Cardiac Pacemakers in a Nationwide Assessment. JACC Clin Electrophysiol 2017;3:1296-305. [Crossref]
- Mazine A, Teoh K, Bouhout I, et al. Sutureless Aortic Valve Replacement: A Canadian Multicentre Study. Can J Cardiol 2015;31:63-8. [Crossref] [PubMed]
- Mazine A, Bonneau C, Karangelis D, et al. Sutureless aortic valves: who is the right patient? Curr Opin Cardiol 2017;32:130-6. [PubMed]
- Margaryan R, Kallushi E, Gilmanov D, et al. Sutureless Aortic Valve Prosthesis Sizing: Estimation and Prediction Using Multidetector-Row Computed Tomography. Innovations (Phila) 2015;10:230-5. [Crossref] [PubMed]
Cite this article as: Chauvette V, Mazine A, Bouchard D. Ten-year experience with the Perceval S sutureless prosthesis: lessons learned and future perspectives. J Vis Surg 2018;4:87.