Total aortic repair for acute type A aortic dissection: a new paradigm
Introduction
The current most widely practised technique for the management of acute type A aortic dissection (ATAAD) consists of replacement of the ascending aorta and “hemi-arch” with an open distal anastomosis, together with either aortic valve resuspension and obliteration of the aortic root false lumen or a Bentall’s procedure. The implicit assumption is that this prevents aortic rupture, aortic regurgitation, and coronary ischemia while removing the primary tear and restoring antegrade true lumen perfusion. The belief is that this procedure is the most likely to yield a live patient, even in the hands of inexperienced cardiac surgeons in the middle of the night. Furthermore, it is assumed that any future aortic complications are very uncommon, and if they occur are very delayed and can be managed in experienced aortic centres with acceptably low morbidity and mortality. These noble intentions are difficult to argue against, but what evidence do we have that these assumptions are correct? May a seemingly more extensive procedure which more completely and reliably corrects the underlying pathology, yield better outcomes both in the early phase as well as the longer term?
How good is the conservative approach?
The latest college guidelines are rather vague in their recommendations. The 2010 American guidelines state “…all of the aneurysmal aorta and the proximal extent of the dissection should be resected… (Class IC)” (1). The 2014 European guidelines recommend “…ascending aortic replacement or hemiarch replacement alone is technically easier.”, but recognise “…Patients with visceral or renal malperfusion..might profit from extended therapies, such as ‘frozen elephant trunk’…” (2)
The logical way to assess any surgical philosophy in the treatment of ATAAD, is by evaluating its acute safety as a salvage procedure and its ability to bring the patient’s long-term outlook back to that of the underlying population.
Acute results
Notwithstanding the recent strides in surgical tools and intensive care, the mortality of ATAAD has remained at 17–26% in the IRAD registry (3). Understandably, the acute salvage is strongly influenced by the occurrence and extent of pre-operative complications such as shock and various organ malperfusion, as conveniently summarised by the Pennsylvania University classification (4). Nevertheless, this cannot be the whole explanation, as demonstrated in a series of 360 patients operated on for ATAAD from Olsson et al. (5), where the mortality was still 14% in the most frequent (61%) and lowest risk (Penn Aa) subgroup, where one would have expected an outcome approaching that of an elective operation.
It is not difficult to envisage why a more conservative operation may fail to achieve its goals. Namely persistence of false lumen pressurisation. In the aortic root, this can result in severe aortic regurgitation and coronary ischemia, while distally it may result in organ or limb ischemia. Finally, in either direction, it can lead to disruption of fresh suture lines with uncontrollable bleeding.
Medium and long-term outcomes
If the long-term fate of the distal aorta after the open distal anastomosis “hemi-arch” repair for ATAAD is examined superficially, the outcomes seem not too pessimistic with 5- and 10-year survival of 70–80% and 50–65% respectively, and a distal re-operation rate of 5–15% and 15–25% at 5 and 10 years (6-8). However, a more careful analysis reveals a very different picture. Firstly, it is vital to make the distinction between type A’s with versus those without involvement of the descending aorta at the initial event. Those with dissection confined to the ascending with or without the arch (“extended” De Bakey Type II) and representing around 35% of all ATAAD, clearly have a much better outlook with 5-year survival and freedom from distal re-intervention of 80% and 100% respectively, versus 63% and 53% in those with Type I ATAAD (9). Secondly, persistence of false lumen (FL) patency portends worse 10-year survival (60% vs. 90%) and freedom from distal re-intervention (64% vs. 94%) (10). Thirdly, re-intervention, while it can be performed with very good outcomes in experienced aortic centres (11), still carries significant complications compared to equivalent first time aortic surgery in those institutions, let alone in the hands of the average cardiac surgeon. Even totally endovascular re-interventions carry significant risks of not only mortality, but stroke and paraplegia and are plagued by endoleaks (12). Finally, and most disturbingly, many of the aortic ruptures occurred in patients under close and regular surveillance (7).
Mechanism of false lumen growth
Putting aside the favourable subgroup with either De Bakey Type II ATAAD or thrombosed FL, we clearly have room for improving acute and chronic outcomes in the proper Type I cases with patent distal FL.
It is essential to go back to basics and try to understand the mechanism behind FL patency and its continued growth. There are the usual factors such as a weakened wall consisting of fewer layers, LaPlace’s law acting on a larger total external diameter of the aorta compared to prior to the dissection and dependence of some branches for their blood supply from the FL. While these are definitely important factors, a major component is a newly created unfavourable hemodynamic situation for the FL in some situations. These tend to be in cases where there is a large proximal descending aortic re-entry tear (which becomes the new entry tear after the surgical repair of the proximal aorta), and small distal re-entry tears. This creates a situation where blood enters the FL freely during systole, but has a hard time emptying itself in diastole through the serpiginous and small distal tears, compared to the rapid distal run off in the true lumen (TL) (13). Hence the mean pressure in the FL is greater than would be expected from systemic blood pressure measurements. This explains the seemingly paradoxical situation where partial thrombosis of the FL has a worse outcome than a completely patent one (14). Here, the thrombus is not only a marker of slow flow associated with poor drainage of FL, but also aggravates the situation by further reducing the size of the diastolic egress, making the FL even more hypertensive. Added to this are the high shear forces from turbulence in the FL near the proximal entry site (15).
How can we improve the early and late results? Understanding the underlying mechanisms, there are two potential modes of attack.
Obliterate large proximal entry sites
Total arch replacement (TAR)
Performing a routine TAR in ATAAD, has the potential to remove more intimal tears apart from the primary tear. It may also capture more of the very proximal descending tears. This has been reasonably criticised as possibly carrying a higher early complication rate in the acute setting. Nonetheless, several current series including ours have reported excellent outcomes (16,17). A recent large meta-analysis has not shown an increase in mortality with TAR (18), so the acute results are not objectively worse. Unfortunately, the analysis also failed to show any significant reduction in long-term distal aortic events. This is not altogether surprising, given that the extra volume of aorta and number of re-entry sites removed in TAR compared to hemi-arch is small compared to the amount remaining in the residual thoraco-abdominal aorta.
Nonetheless, the added benefits of TAR go beyond. Firstly, when combined with appropriate management of the root, TAR minimises the need for a difficult re-sternotomy to treat residual disease in the proximal aorta and arch. Secondly, providing a Dacron long “landing zone” away from the arch branches greatly increases the facility and options of future endovascular treatment.
Total arch replacement and “Frozen Elephant Trunk”
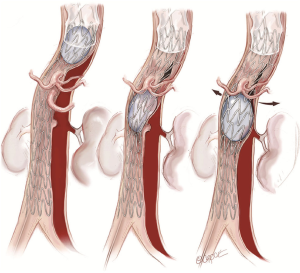
Extending the length of descending aorta whose intimal tears are sealed, either with an open or endovascular stent graft, can be reasonably expected to improve the outlook for the distal aorta. Indeed, if one examines the segment of aorta treated, there is more than 90% chance of achieving thrombosis and shrinkage of the FL (19). Disappointingly, this beneficial effect tended to be confined to the stented segment, with few obtaining positive remodeling in the lower thoracic and abdominal aorta. The reasons for this include large distal re-entries, especially at visceral branches or iliacs, and new intimal tears from the trauma of the distal edge of the stent on the fragile intima (Figure 1: 1b and 3b). On the other side of the equation, there have been disturbing reports of 7.5% procedural incidence of paraplegia (20), a hitherto rare complication of ATAAD repair, especially when prolonged periods of circulatory arrest without antegrade cerebral perfusion and higher perfusion temperatures, and elephant trunks extending beyond T9 are used. Furthermore, if future procedures are required on the distal thoraco-abdominal aorta, the complexity and risks are still significant.
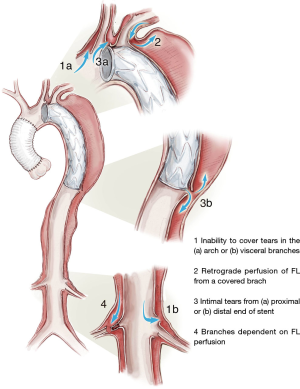
Distal fenestration
Localised
The percutaneous creation of a more generous re-entry was first described in 1990 (21). Once a communication between true and false channels is created with a wire, it can be enlarged with a balloon or even a stent. This can be quite effective in relieving TL collapse in the vicinity of an ischaemic visceral artery. This approach may be quite useful for the rapid relief of organ ischemia, but is generally not sufficient to ensure complete decompression of the FL.
Extensive
A more extensive fenestration can be very effectively done surgically and has been shown to give good long-term outcomes not only to relieve organ ischaemia, but also to prevent aortic aneurysm formation (22). Nonetheless, the open procedure is a significantly invasive procedure. A percutaneous “cheese wire” technique for creating a long fenestration has been described (23), but is technically challenging and has had limited application. A more facile technique involves the initial placement of bare metal stents in the lower thoracic and whole of the abdominal TL, which are then balloon expanded to produce a longitudinal and complete fenestration (24).
The paradigm shift: total aortic repair for ATAAD
Utilising the preceding deliberations, we have arrived at a novel formulation for the management of ATAAD, with the desire of combining a safe acute operation together with the potential for achieving long-term healing of the entire aorta, with minimal future complications or need for re-intervention.
Stage 1
The majority of patients presenting with ATAAD will proceed to a “Branch First” total aortic arch repair as previously described by us (25). Essentially this consists of sequential short period clamping and re-perfusion of the 3 arch branches, without circulatory arrest and using a modified trifurcation graft with a side perfusion port (Figure 2).
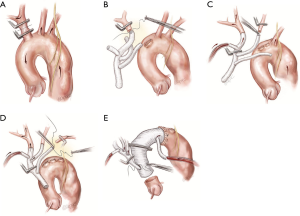
The distal anastomosis can be performed with a cross clamp allowing distal organ perfusion to continue, although if there is any concern about quality of distal aortic wall or potential for distal malperfusion, we don’t hesitate to use a short period of distal circulatory arrest with open distal anastomosis. It is essential to construct the trifurcation to main Dacron graft anastomosis sufficiently proximally, to allow a long “landing zone” for subsequent endovascular stenting.
We don’t routinely use The FET technique unless we have concern about the proximal descending aorta being very fragile, or containing a primary tear with retrograde ATAAD, or a large re-entry tear with pre-operative distal mal-perfusion.
Liberal use is made of valve sparing root replacement or Bentall’s procedure when appropriate.
Stage 2
The decision to proceed to stenting of the remaining dissected thoracoabdominal aorta is dependent on clinical and imaging grounds. In the uncommon situation of a patient presenting with advanced visceral ischaemia, stenting is performed preoperatively allowing time for metabolic derangements to be corrected before ATAAD repair. Stenting has also been undertaken immediately after the completion of ATAAD repair in a few cases, for persistent malperfusion despite redirection of flow to the TL proximally. The majority have undergone the second stage either after discharge from ICU or on an early readmission. The indications for these included evolving malperfusion, a rapidly enlarging false lumen on CTA, or collapsed true lumen.
Stenting of the remaining dissected thoracoabdominal aorta proceeds as follows. In the angiography suite, a covered endovascular stent is placed with a generous overlap proximally to the previously set up long landing zone, and extending distally to the level of mid descending aorta (Figure 3)
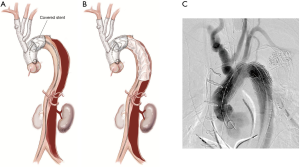
Uncovered dissection stents (Zenith Dissection endovascular stent, Cook Medical Inc., Bloomington, IN, USA) are then deployed through the remaining thoracoabdominal aorta to the bifurcation (Figure 4). Stent graft overlap in the visceral aorta is avoided as this can make it difficult to wire visceral vessels if branch stenting is required. The stents are then balloon dilated (Coda Balloon Catheter, Cook Medical Inc., Bloomington, IN, USA) to rupture the septum between true and false lumens. Balloon dilatation begins at the overlap between covered and uncovered stents and proceeds distally (Figure 5). Successful rupture of the septum is indicated by a “popping” sensation on the end of the syringe and a sudden enlargement of the TL, which doesn’t recoil back after balloon deflation (Figure 6). This serves the purpose of creating a single aortic channel, expanding the true lumen completely and decompressing the false lumen to appose the layers of the aorta, allowing healing of the layers and aortic remodelling. In the medium to long term, it removes the chronic driving force behind false lumen growth and combined with the structural support to the wall that the stents provide, reduces aneurysmal dilatation. This is much easier to achieve in the acute setting before the septum wall becomes stiff and unyielding, making it difficult to fenestrate. It is important not to inflate the balloon to a diameter greater than the maximum aortic diameter of that segment as measured on CTA, as this may lead to aortic rupture.
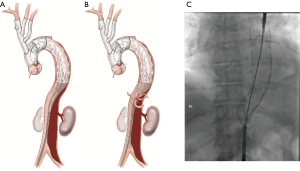

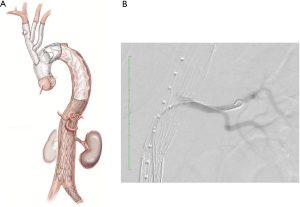
If there is compromise to the visceral vessels which have some supply from the false lumen (particularly the renal arteries), it is pertinent to place a wire into these vessels before inflation to ensure timely access to the vessel should perfusion be compromised with balloon inflation. In these cases, a covered visceral branch vessel stent can be deployed to re-establish flow to the compromised vessel (Figure 7).
Contraindications to balloon inflation include the presence of leak or suspicion of contained rupture with periaortic haematoma, or subacute dissection with large volume of false lumen thrombus leading to the risk of embolism.
Finally, aortography is performed to confirm patency of the aorta and visceral vessels and obliteration of false lumen flow (Figure 8).

Early experience
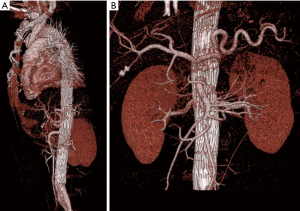
Over the past 5 years, in the setting of ATAAD, total aortic repair by the techniques described above has been performed in 15 patients with an age range of 19–74 years old. Twelve of these patients (80%) have undergone total aortic repair in the acute setting with thoracoabdominal stenting performed within 14 days of initial surgery. There have been no operative or 30-day mortalities in this group with procedural success achieved in all patients as defined by uniluminal flow and reversal of malperfusion. There has been one permanent neurological event after stenting with minor residual deficit and no instances of paraplegia or access complications. Follow up CTA in the medium term has demonstrated no residual false lumen flow, aneurysm or dissection (Figure 9).
Conclusions
Replacement of the ascending aorta and hemi arch with an open distal anastomosis has long been the standard in the management of ATAAD. It is seen as simple and reproducible and the most likely to yield a live patient, especially in inexperienced hands. However, this conservative approach may expose the patient to the acute complications of malperfusion and to a more complex and risky situation in the medium to long term. A more radical approach to ATAAD, if it can be performed safely, would minimise the acute hazards and risks of future interventions. It has been demonstrated that repair of ATAAD involving more extensive replacement of the arch can be performed safely in the acute setting. The Branch-First arch replacement technique allows safe and controlled replacement of the arch with a long Dacron landing zone for subsequent thoracoabdominal stenting. By treating the whole dissected aorta, we aim to obliterate the false lumen and ensure true lumen flow, preventing the acute complications of branch vessel malperfusion while also reducing the incidence of late aneurysmal formation. Although long term follow up is still required, and in particular, outcomes of stent graft treatment of patients with aortopathies remains unknown, we believe that total aortic repair in suitable patients with ATAAD has the potential to improve early and late outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Berretta P, Patel HJ, Gleason TG, et al. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg 2016;5:346-51. [Crossref] [PubMed]
- Augoustides JG, Szeto WY, Desai ND, et al. Classification of acute type A dissection: focus on clinical presentation and extent. Eur J Cardiothorac Surg 2011;39:519-22. [Crossref] [PubMed]
- Olsson C, Hillebrant CG, Liska J, et al. Mortality in acute type A aortic dissection: validation of the Penn classification. Ann Thorac Surg 2011;92:1376-82. [Crossref] [PubMed]
- Geirsson A, Bavaria JE, Swarr D, et al. Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 2007;84:1955-64; discussion 1955-64.
- Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35. [Crossref] [PubMed]
- Kimura N, Tanaka M, Kawahito K, et al. Influence of patent false lumen on long-term outcome after surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2008;136:1160-6, 1166.e1-3.
- Tsagakis K, Tossios P, Kamler M, et al. The DeBakey classification exactly reflects late outcome and re-intervention probability in acute aortic dissection with a slightly modified type II definition. Eur J Cardiothorac Surg 2011;40:1078-84. [PubMed]
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50. [Crossref] [PubMed]
- Bajona P, Quintana E, Schaff HV, et al. Aortic arch surgery after previous type A dissection repair: results up to 5 years. Interact Cardiovasc Thorac Surg 2015;21:81-5. [Crossref] [PubMed]
- Spear R, Hertault A, Van Calster K, et al. Complex endovascular repair of postdissection arch and thoracoabdominal aneurysms. J Vasc Surg 2018;67:685-93. [Crossref] [PubMed]
- Berguer R, Parodi JC, Schlicht M, et al. Experimental and clinical evidence supporting septectomy in the primary treatment of acute type B thoracic aortic dissection. Ann Vasc Surg 2015;29:167-73. [Crossref] [PubMed]
- Tsai MT, Wu HY, Roan JN, et al. Effect of false lumen partial thrombosis on repaired acute type A aortic dissection. J Thorac Cardiovasc Surg 2014;148:2140-6.e3. [Crossref] [PubMed]
- Alimohammadi M, Sherwood JM, Karimpour M, et al. Aortic dissection simulation models for clinical support: fluid-structure interaction vs. rigid wall models. Biomed Eng Online 2015;14:34. [Crossref] [PubMed]
- Glauber M, Murzi M, Farneti P, et al. Aortic arch replacement with prophylactic aortic arch debranching during type A acute aortic dissection repair: initial experience with 23 patients. Eur J Cardiothorac Surg 2011;40:418-23. [PubMed]
- Matalanis G, Perera NK, Galvin SD. Aortic arch replacement without circulatory arrest or deep hypothermia: the "branch-first" technique. J Thorac Cardiovasc Surg 2015;149:S76-82. [Crossref] [PubMed]
- Poon SS, Theologou T, Harrington D, et al. Hemiarch versus total aortic arch replacement in acute type A dissection: a systematic review and meta-analysis. Ann Cardiothorac Surg 2016;5:156-73. [Crossref] [PubMed]
- Iafrancesco M, Goebel N, Mascaro J, et al. Aortic diameter remodelling after the frozen elephant trunk technique in aortic dissection: results from an international multicentre registry. Eur J Cardiothorac Surg 2017;52:310-8. [Crossref] [PubMed]
- Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J Cardiothorac Surg 2016;49:660-6. [Crossref] [PubMed]
- Williams DM, Brothers TE, Messina LM. Relief of mesenteric ischemia in type III aortic dissection with percutaneous fenestration of the aortic septum. Radiology 1990;174:450-2. [Crossref] [PubMed]
- Trimarchi S, Segreti S, Grassi V, et al. Open fenestration for complicated acute aortic B dissection. Ann Cardiothorac Surg 2014;3:418-22. [PubMed]
- Watkinson AF. A novel "cheese wire" technique for stent positioning following difficult iliac artery subintimal dissection and aortic re-entry. Cardiovasc Intervent Radiol 2009;32:781-4. [Crossref] [PubMed]
- Hofferberth SC, Nixon IK, Boston RC, et al. Stent-assisted balloon-induced intimal disruption and relamination in aortic dissection repair: the STABILISE concept. J Thorac Cardiovasc Surg 2014;147:1240-5. [Crossref] [PubMed]
- Matalanis G, Galvin SD. "Branch-first" continuous perfusion aortic arch replacement and its role in intra-operative cerebral protection. Ann Cardiothorac Surg 2013;2:194-201. [PubMed]
- Galvin SD, Perera NK, Matalanis G. Surgical management of acute type A aortic dissection: branch-first arch replacement with total aortic repair. Ann Cardiothorac Surg 2016;5:236-44. [Crossref] [PubMed]
- Matalanis G, Ip S. Balloon dilation of bare metal stent demonstrating rupture of septum. Asvide 2018;5:411. Available online: http://www.asvide.com/article/view/24377
- Matalanis G, Ip S. Final angiography demonstrating true lumen flow with no filling of false lumen. Asvide 2018;5:412. Available online: http://www.asvide.com/article/view/24378
Cite this article as: Matalanis G, Ip S. Total aortic repair for acute type A aortic dissection: a new paradigm. J Vis Surg 2018;4:79.


