Cerebral perfusion issues in type A aortic dissection
Introduction
There is no doubt that adequate cerebral protection during any kind of arch surgery plays a key role in achieving successful outcomes. Aortic arch reconstruction in type A acute aortic dissection (TAAAD) always requires to protect the brain from ischaemic and embolic injury, to ensure patency of the brachiocephalic vessels with reduced trauma to these friable vessels, and to restore the blood flow in to the true lumen performing a durable distal anastomosis.
Currently, it has been largely demonstrated that optimizing cerebral protection with hypothermia and adjunctive cerebral perfusion allows for safe, extended periods of circulatory arrest and open arch reconstruction (1-3).
However, neurological injuries still represent a relevant issue in aortic dissection because the frequency of both preoperative and postoperative cerebral events is high and negatively impact the prognosis of these patients. A recent observational International Registry of Acute Aortic Dissection (IRAD) study, clearly reported as almost one third of the 2,400 patients with TAAAD included in the analysis suffered from pre or postoperative neurological deficit as cerebral vascular accident, coma or even spinal cord injury (4).
What we would like to know when we deal with TAAAD is how much the technique influences neurological outcomes and how much the dissection itself impairs cerebral functions. Many times patients are referred for emergent operations but despite the lack of a clear evidence of cerebral malperfusion they develop neurological symptoms during the post-operative course, and it is also true the opposite, even less frequently, patients with clear signs of cerebral malperfusion do not develop permanent neurological deficits postoperatively. This is because we don’t know how much malperfusion already impaired neurological functions (Figure 1).
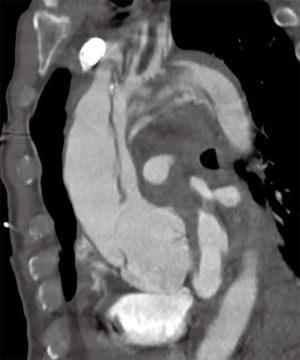
The aim of the current review article is to go over the primary open issues and state of the art for what concern cerebral protection during surgery for TAAAD.
Literature search criteria
Selection of literature articles was performed using PubMed databases from inception to February 2018, using ‘surgical treatment of type A aortic dissection’ OR ‘neurological events in aortic dissection’ OR ‘cerebral protection in aortic dissection’ OR ‘malperfusion in type A aortic dissection’ OR ‘neurological events after aortic dissection’ as either keywords or MeSH terms. Case reports, editorial, expert opinion and comment types of publication were excluded as well as review articles because of potential doubling of results. Among series coming from the same group only the most recent ones were considered. Primary endpoints included pre-operatory neurological events, in-hospital death, cerebral protection strategy and postoperative permanent and permanent neurologic dysfunction.
Results
In Table 1, it summarized observational single center experience focusing on neurological outcomes after TAAAD (5-15).
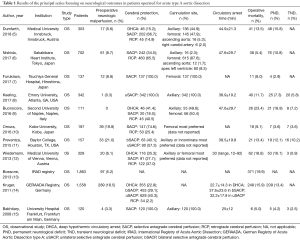
Full table
Among the 11 studies identified in this review, antegrade cerebral perfusion (ACP) was the preferred method of cerebral protection ranging from 28% to 100% between different strategies, followed by retrograde cerebral perfusion (RCP) and deep hypothermic circulatory arrest (DHCA) alone. Operative mortality and permanent neurological deficits varies among 5% to 23% and 3% to 19%, respectively.
According to the data from the German Registry of Acute Aortic Dissection type A (GERAADA) 18.5% of 1,558 patients included in the study presented neurologic deficits at presentation (14). Cerebral protection was achieved with hypothermic circulatory arrest alone in 355 (22.8%), unilateral ACP in 628 (40.3%), bilateral ACP (bACP) in 453 (29.1%), and retrograde perfusion in 34 patients (2.2%). Hypothermic circulatory arrest alone resulted in a mortality-corrected permanent neurological dysfunction rate of 11.5%, whereas the rate was 10.0%, for unilateral ACP and 11.0% for bACP. For hypothermic circulatory arrest times superior to 30 minutes there was a profound increase in mortality compared with the ACP groups (P<0.001) (14).
The data presented by Bossone et al. (13) from the multicenter IRAD determined the incidence and prognostic impact of stroke in 2,202 patients who were treated either surgically or medically. Stroke was present at arrival in 132 (6.0%) patients. These patients were older (65±12 vs. 62±15 years; P=0.002) and presented more often with shock (14% vs. 7%; P=0.005) and arch vessel involvement at angio-CT (68% vs. 37%; P<0.001) than patients without stroke. As expected, In-hospital complications and mortality [odds ratio (OR) =1.62]were higher among patients with stroke; however, surgical management had a strong independent association with improved survival compare to medical treatment alone (13).
The latest series available on this topic, investigated the predictors and outcomes of stroke in 303 patients with TAAAD undergoing surgical repair (5). The prevalence of permanent postoperative neurologic injuries was 15.8% and multivariable analysis identified the presence of bovine aortic arch (OR =2.33), preoperative cardiopulmonary resuscitation (OR =6.483) and preoperative malperfusion (OR =2.536) as independent predictors for postoperative stroke. The occurrence of stroke had a strong impact on morbidity and was associated with higher rates of postoperative complications and a significantly longer hospital stay (stroke: 23±16 days vs. no stroke: 17±18 days; P=0.021) (5).
Discussion
Management of cerebral malperfusion
Historically, brain injury at presentation adversely affected hospital survival of patients with TAAAD (16). Of 1,873 patients with type A dissection enrolled in IRAD, 87 (4.6%) presented with cerebrovascular accident and 54 (2.9%) with coma (17). These patients were more likely to have shock, hypotension, or tamponade (46.8% vs. 25.2%) and arch vessel involvement (55.0% vs. 36.1%). IRAD investigators studied patients by the presence and type of brain injury (no injury vs. stroke vs. coma) and by management treatment (medical vs. surgical). Indeed, the presence of brain injury significantly affected the therapeutic management. Surgery was not performed in 11% of patients without brain injury, 24.1% of patients with cerebrovascular accident, and 33.3% of patients with coma. The reason is likely because cerebral reperfusion and hemorrhagic conversion of the ischemic region might worsen neurologic outcomes and lead to prohibitive postoperative mortality and morbidity rates (16,18).
However, when assessing hospital outcomes according to therapeutic management the authors showed that medical therapy was associated with dismal outcomes: 100% mortality in patients with coma and 76.2% in those with cerebrovascular accident (17). However, surgery led to a hospital survival benefit of 49.6% in patients with preoperative neurologic symptoms and 55.6% of those with coma. Only 12.8% of the medically treated patients with preoperative brain injury survived to discharge compared with 66.7% of those undergoing surgical repair. Moreover 5-year survival of patients presenting with cerebrovascular accident and coma was 23.8% and 0% after medical management vs. 67.1% and 57.1% after surgery (P<0.001).
Czerny and coauthors (19) evaluated the impact of malperfusion syndromes in the GEERADA registry on postoperative neurologic injuries. Out of 2,137 consecutive patients enrolled in the study about 1/3 of the cases experienced signs of pre-operative malperfusion (coronary, cerebral, spinal, visceral, renal, peripheral). The study showed as regardless of pre-operative status, the dissection of supra-aortic branches was an independent predictor of post-operative cerebral injuries with an estimated OR of 2.18.
Indeed, in case of cerebral malperfusion, we still don’t have any definitive evidence that a specific surgical strategy or perfusion technique could ameliorate the prognosis of the patients.
In a remarkable comment for the Journal of the American College of Cardiology, Stewart and Chikwe from the Mount Sinai Hospital in New York suggested to think beyond surgery as in addition to immediate aortic repair, only an aggressive strategies by experienced teams to address ischemia pre-operatively, intra-operatively, or with hybrid therapy should be always considered in this setting (20).
Cerebral protection during aortic arch repair
In order to protect the brain during TAAAD repair, three techniques have been proposed and widely utilized as a means of protecting the brain: DHCA, RCP and antegrade selective cerebral perfusion (ASCP).
However, we are not sure that optimizing cerebral protection allows for safe post-operative outcome. Figure 2 is showing a complex case of TAAAD in a patient with preoperative signs of cerebral and visceral malperfusion. Of course, our task is to give the best treatment available all the time but we still have a large number of issues for which we have incomplete or no responses at all. Open issues regard the site of arterial cannulation during ACP, the possibility to perfuse unilaterally or bilaterally both hemispheres and how to manage patients with cerebral malperfusion.
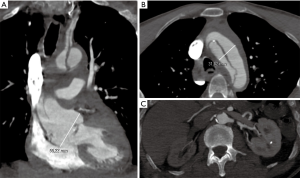
While ACP emerged as the preferred perfusion strategy in many centres, the choice of access site for arterial cannulation remains an ongoing topic.
In a recent European survey on current trends in cannulation and neuroprotection-strategies during surgery of the aortic arch, the right subclavian-axillary approach was the favorite site for arterial cannulation (21). Standardization and simplification of cerebral perfusion has favored the axillary artery over the femoral arterial inflow to be the current main access in the case of aortic dissection. Using this advanced approach is possible to simply switch from standard cardio-pulmonary-bypass to unilateral ACP just clamping the innominate artery at the base (21). Axillary cannulation can be performed either directly or via the interposition of an 8 mm vascular prosthesis to optimize haemostasis and reduce the vessel traumatism.
The latest evidence coming from the IRAD registry (22) showed significant improvements during the last 20 years in the outcomes of TAAAD thanks to the advancement in the surgical techniques and confirmed the ACP using the axillary cannulation to be the preferred latest method of cerebral protection.
Another, site of supra-aortic arterial cannulation is represented by the innominate artery but unfortunately is frequently involved in the dissection and therefore not suitable for cannulation.
Common carotid artery can be an alternative approach, the experience is limited to only few centres worldwide and it is difficult to give general advice (1). However, in selected patients could be really effective. Short series successfully reported this type of approach in patients with TAAAD having signs of malperfusion (23,24). The additional cannulation of occluded or compressed carotid artery was able to quickly reperfuse the brain and ensure at least unilateral unrestricted cerebral perfusion during cardiopulmonary bypass (CPB). In 2016 Okita et al., reported three interesting cases of patients presenting with brain malperfusion secondary to TAAAD who underwent preoperative perfusion of the right common carotid artery before surgical repair (24). They all arrived at hospital in a comatose or semi-comatose state with left hemiplegia. The right common carotid artery was exposed and directly cannulated using a 12-Fr cannula, a bigger 14-Fr double-lumen cannula was chosen for additional arterial drainage of the right femoral artery (24). The circuit contained a small roller pump (target flow was set at 90 mL/min) and heat exchanger coil. Initial results of this brain-saving system were very promising as all patients survived to arch replacement with minimal postoperative neurological sequelae (24).
Unilateral vs. bilateral cerebral perfusion strategy
According to the survey of the European Association for Cardio-Thoracic Surgery (EACTS) vascular domain, bACP is now the most frequent method for brain protection in acute presentation (21). However, in more then 1/3 of the cases, unilateral perfusion was used.
Despite the lack of clear evidences in favor of bilateral cerebral perfusion especially in acute scenarios, some relevant reflections should be advanced. First one in the literature, the series on unilateral cerebral perfusion are referred almost always to short times of cerebral perfusion and, for sure, good results are reported.
In a review analysis of Malvindi et al. the authors identified 17 papers to answer on whether unilateral ACP is safe as bilateral cerebral perfusion during aortic arch surgery (25). ACP was used in a total of 3,548 patients: bilaterally in 2,949 patients and unilaterally in 599 patients. Both methods of cerebral perfusion resulted in neurological injury rates inferior to 5%, but the period of bACP perfusion was significantly higher (86 to over 164 min) compared to the mean duration (32 min) of unilateral cerebral perfusion.
Second issue regarding the open debate on unilateral vs. bilateral cerebral perfusion concern relevant pathophysiologic considerations. One of the most relevant is that cerebral flow gradually declines during cerebral perfusion at each temperature, and this reduction is not the same in every cerebral region (26). This means that there are areas more sensitive to the ischemic injury, like the pons and there are others areas less sensitive, like the hippocampus. Another practical aspect is that: longer is the cerebral perfusion time, higher is the risk of cerebral ischemic injury.
Moreover, we demonstrated (27) with positron emission tomography (PET) scan and magnetic resonance imaging (MRI) that patients undergoing open arch surgery using bilateral cerebral perfusion develop temporary hypometabolism of the occipital lobes as a consequence of an ischemic injury due to the lack of left subclavian artery perfusion and, this hypometabolism is more important and evident for longer period of cerebral perfusion.
In 2017 was published in the Journal of Thoracic and Cardiovascular Surgery the only study comparing unilateral vs. bilateral cerebral perfusion in 203 patients presenting TAAAD (28). There was no significant difference between groups in terms of CPB, cross-clamp and circulatory arrest times. It failed to demonstrate a significant difference between the two techniques; but looking carefully at the data, bilateral perfusion implied 50% less mortality and neurologic morbidity rates than unilateral perfusion.
In one of the two editorials of this paper (29), the author reported a Hippocrates cite saying, “Make a habit of two things: to help; or at least do no harm.” By using bACP, we may achieve both of these goals. Although definitive proof that bACP is superior to unilateral ACP is still lacking: if it does not cause any obvious harm, then why not?
Conclusions
Brain malperfusion caused by acute type A aortic dissection is a silent condition that may not be always clinically evident but impair outcomes regardless of the operative techniques.
Cerebral protection strategies evolved during the years with a clear advantage of ACP using the axillary artery as preferred site of arterial inflow. Although definitive proof that bACP is superior to unilateral ACP is still lacking, there is any obvious harm to use a complete brain protection especially when prolonged periods of ACP are anticipated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rylski B, Urbanski PP, Siepe M, et al. Operative techniques in patients with type A dissection complicated by cerebral malperfusion. Eur J Cardiothorac Surg 2014;46:156-66. [Crossref] [PubMed]
- Cefarelli M, Murana G, Surace GG, et al. Elective Aortic Arch Repair: Factors Influencing Neurologic Outcome in 791 Patients. Ann Thorac Surg 2017;104:2016-23. [Crossref] [PubMed]
- Pacini D, Di Marco L, Leone A, et al. Cerebral functions and metabolism after antegrade selective cerebral perfusion in aortic arch surgery. Eur J Cardiothorac Surg 2010;37:1322-31. [Crossref] [PubMed]
- Isselbacher EM, Bonaca MP, Di Eusanio M, et al. Recurrent Aortic Dissection: Observations From the International Registry of Aortic Dissection. Circulation 2016;134:1013-24. [Crossref] [PubMed]
- Dumfarth J, Kolfer M, Stastny L, et al. Stroke after emergent surgery for acute type A aortic dissection: predictors, outcome and neurological recovery. Eur J Cardiothorac Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Nishida H, Tabata M, Fukui T, et al. A systematic approach to improve the outcomes of type A aortic dissection. J Thorac Cardiovasc Surg 2017;154:89-96.e1. [Crossref] [PubMed]
- Furukawa T, Uchida N, Takahashi S, et al. Management of cerebral malperfusion in surgical repair of acute type A aortic dissection. Eur J Cardiothorac Surg 2017;52:327-32. [Crossref] [PubMed]
- Keeling WB, Leshnower BG, Hunting JC, et al. Hypothermia and Selective Antegrade Cerebral Perfusion Is Safe for Arch Repair in Type A Dissection. Ann Thorac Surg 2017;104:767-72. [Crossref] [PubMed]
- Buonocore M, Amarelli C, Scardone M, et al. Cerebral perfusion issues in acute type Aaortic dissection without preoperative malperfusion: how do surgical factors affect outcomes? Eur J Cardiothorac Surg 2016;50:652-9. [Crossref] [PubMed]
- Omura A, Miyahara S, Yamanaka K, et al. Early and late outcome of repaired acute DeBakey type I aortic dissection after graft replacement. J Thorac Cardiovasc Surg 2016;151:341-8. [Crossref] [PubMed]
- Preventza O, Simpson KH, Cooley DA, et al. Unilateral versus bilateral cerebral perfusion for acute type A aortic dissection. Ann Thorac Surg 2015;99:80-7. [Crossref] [PubMed]
- Wiedemann D, Kocher A, Dorfmeister M, et al. Effect of cerebral protection strategy on outcome of patients with Stanford type A aortic dissection. J Thorac Cardiovasc Surg 2013;146:647-55.e1. [Crossref] [PubMed]
- Bossone E, Corteville DC, Harris KM, et al. Stroke and outcomes in patients with acute type A aortic dissection. Circulation 2013;128:S175-9. [Crossref] [PubMed]
- Kruger T, Weigang E, Hoffmann I, et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation 2011;124:434-43. [Crossref] [PubMed]
- Bakhtiary F, Dogan S, Zierer A, et al. Antegrade cerebral perfusion for acute type A aortic dissection in 120 consecutive patients. Ann Thorac Surg 2008;85:465-9. [Crossref] [PubMed]
- Cambria RP, Brewster DC, Gertler J, et al. Vascular complications associated with spontaneous aortic dissection. J Vasc Surg 1988;7:199-209. [Crossref] [PubMed]
- Di Eusanio M, Patel HJ, Nienaber CA, et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg 2013;145:S213-21.e1. [Crossref] [PubMed]
- Pocar M, Passolunghi D, Moneta A, et al. Recovery of severe neurological dysfunction after restoration of cerebral blood flow in acute aortic dissection. Interact Cardiovasc Thorac Surg 2010;10:839-41. [Crossref] [PubMed]
- Czerny M, Schoenhoff F, Etz C, et al. The Impact of Pre-Operative Malperfusion on Outcome in Acute Type A Aortic Dissection: Results From the GERAADA Registry. J Am Coll Cardiol 2015;65:2628-35. [Crossref] [PubMed]
- Stewart A, Chikwe J. Beyond the tube graft. Thinking Beyond the Tube Graft: Using Malperfusion as a Guide to Define Treatment of Type A Dissection. J Am Coll Cardiol 2015;65:2636-7. [Crossref] [PubMed]
- De Paulis R, Czerny M, Weltert L, et al. Current trends in cannulation and neuroprotection during surgery of the aortic arch in Europe. Eur J Cardiothorac Surg 2015;47:917-23. [Crossref] [PubMed]
- Parikh N, Trimarchi S, Gleason TG, et al. Changes in operative strategy for patients enrolled in the International Registry of Acute Aortic Dissection interventional cohort program. J Thorac Cardiovasc Surg 2017;153:S74-9. [Crossref] [PubMed]
- Urbanski PP. Carotid artery cannulation in acute aortic dissection with malperfusion. J Thorac Cardiovasc Surg 2006;131:1398-9. [Crossref] [PubMed]
- Okita Y, Matsumori M, Kano H. Direct reperfusion of the right common carotid artery prior to cardiopulmonary bypass in patients with brain malperfusion complicated with acute aortic dissection. Eur J Cardiothorac Surg 2016;49:1282-4. [Crossref] [PubMed]
- Malvindi PG, Scrascia G, Vitale N. Is unilateral antegrade cerebral perfusion equivalent to bilateral cerebral perfusion for patients undergoing aortic arch surgery? Interact Cardiovasc Thorac Surg 2008;7:891-7. [Crossref] [PubMed]
- Strauch JT, Haldenwang PL, Müllem K, et al. Temperature dependence of cerebral blood flow for isolated regions of the brain during selective cerebral perfusion in pigs. Ann Thorac Surg 2009;88:1506-13. [Crossref] [PubMed]
- Pacini D, Di Marco L, Leone A, et al. Cerebral functions and metabolism after antegrade selective cerebral perfusion in aortic arch surgery. Eur J Cardiothorac Surg 2010;37:1322-31. [Crossref] [PubMed]
- Tong G, Zhang B, Zhou X, et al. Bilateral versus unilateral antegrade cerebral perfusion in total arch replacement for type A aortic dissection. J Thorac Cardiovasc Surg 2017;154:767-75. [Crossref] [PubMed]
- Bachet J. I have only 1 brain but 2 hemispheres: Please perfuse both adequately! J Thorac Cardiovasc Surg 2017;154:765-6. [Crossref] [PubMed]
Cite this article as: Pacini D, Murana G, Di Marco L, Berardi M, Mariani C, Coppola G, Fiorentino M, Leone A, Di Bartolomeo R. Cerebral perfusion issues in type A aortic dissection. J Vis Surg 2018;4:77.


