Type B aortic dissection: new perspectives
Classifications of type B aortic dissections (tBAD)
tBAD, per definition affecting the aorta distal to the left subclavian artery and not affecting the aortic arch and ascending aorta, can be classified as acute or chronic depending on the onset of symptoms of the dissection. When the time between the onset of the acute symptoms and the diagnosis is less than 2 weeks, the dissection is classified as acute. This classic definition is based on autopsy studies of patients with aortic dissection of any type, showing that 74% of the deaths from complications of dissection occurred within 2 weeks. This definition is now used in trials and in everyday clinical practice. Actually however some studies have shown that a significant proportion of patients presenting with acute complications require endovascular treatment 15–85 days after onset of aortic dissection (1). This indicates that there is a sub-acute, unstable phase between 2 weeks and 3 months in the transition between acute and chronic dissection during which acute and life-threatening complications might occur which questions the relevance of the current definition. Based on time frame, the International Registry of Aortic Dissection (IRAD) investigators subclassify aortic dissection patients as hyperacute (symptom onset up to 24 hours), acute (2–7 days), subacute (8–30 days), and chronic (>30 days) (2). The division in time between these types is important with respect to the gradual loss of plasticity of the intimal septum over time and the decreasing possibilities to remodel the dissection.
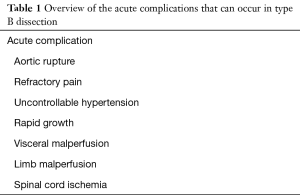
Another very important classification of tBAD concerns the presence or occurrence of complications and this allows us to differentiate between uncomplicated and complicated type B’s. This is important because hospital mortality is approximately 50% in complicated versus only 10% in uncomplicated patients. Classically acute complications are defined as frank rupture, impending rupture, refractory pain, persistent and uncontrollable hypertension despite adequate medical treatment, rapid growing of the diameter of the dissected aorta, occurrence of acute hoarseness, malperfusion of the viscera or limbs and they are summarized in Table 1. However, some of these items need further clarification. Frank rupture into the pleural cavity will result in an immediate and massive hemothorax leading to deep hypovolemic shock and death. However almost all acute tBAD show some moderate amount of pleural fluid, mostly on the left sight. This implies that a moderate amount of pleural fluid should not be interpreted as a ruptured tBAD; growing mediastinal hematoma or peri-aortic hematoma are signs of impending rupture (so-called contained rupture). Rapid growth means a minimal increase of 0.5 cm per year and should be documented by serial scans. It is hardly understandable that actually hypertension and pain cannot be adequately treated with all existing modern pharmacological means. So, one should be very cautious with labeling hypertension or pain as untreatable. Occlusion of celiac trunk, superior mesenteric, inferior mesenteric and/or renal arteries results in severe abdominal pain and decreased urine output leading to metabolic shock later on. When the flow in the distal abdominal aorta or iliac arteries is compromised, patients may complain of painful, pulseless or even plegic and cold lower extremities. Paraplegia is a devastating consequence of obstruction of critical intercostals and/or lumbar arteries resulting in reduced blood flow in the anterior spinal arteries of the spinal cord. Uncomplicated tBAD refers to stable patients lacking all of these symptoms at presentation and during the complete hospital stay.
Full table
The Dissect taxonomy of aortic dissection not only takes into account the time frame (acute, subacute and chronic) but also the location of the intimal tear, the size of the aorta, the segmental extent of aortic involvement, the presence of complications, and the status of the false lumen (patent, partially or completely thrombosed) (3). It can serve as a guide to support critical analysis of contemporary therapeutic options. The stratification of dissections in acute, subacute, chronic, complicated and uncomplicated will help us to discern which of the patients need intervention or not.
Best medical treatment
All dissections should be treated initially with intravenous anti-impulsive medication as described by Wheat (4) in the early 70’s. In acute circumstances the goal of this treatment is to lower the blood pressure which is increased due to the intense pain. Patients with an acute tBAD are anxious and in distress. Appropriate analgesics (e.g., morphine) should be administered promptly. The target systolic pressure should be 100–120 mmHg, so-called permissive hypotension. The decrease of the dP/dt is an important feature of betablockers together with blood pressure and heart rate control: therefore, betablockers are still the first line drugs. The medical management of uncomplicated tBAD is the standard of care with a lower mortality compared to open surgery. It still remains the cornerstone of modern medical management of tBAD. When hypertension persists despite the use of betablockers, calcium channel blockers can be added. A proposed treatment protocol is described in detail by Tran (5). In chronic circumstances medical treatment consists only of oral medication but with the same goal: avoiding arterial hypertension, promoting aortic stability and preventing aortic expansion that might cause future rupture or recurrent dissection. Besides oral medication long-term blood pressure regulation with adequate aortic surveillance and imaging at regular time intervals is also of vital importance. A very comprehensive overview about the medical treatment of tBAD including the guidelines and findings of the IRAD is given by Suzuki et al. (6).
Treatment modalities of type B aortic dissection
Acute complicated tBAD’s were initially treated with open surgery consisting of the replacement of a part of the dissected thoracic aorta with a vascular Dacron prosthesis. Dr. Cooley and DeBakey were pioneers in this intervention (7). These complex operations had a high mortality due to the acute presentation, the bad hemodynamic conditions and the difficult technical details of these interventions based on the fragility of the aortic layers. Often patients were in deep shock before entering the operating theatre. The quality of the acute dissected aorta demands meticulous surgery using special techniques reinforcing the dissected aortic layers. Also, organ protection poses a typical problem as well as avoidance of bleeding. Not surprisingly results were bad with a high mortality and morbidity.
Since the introduction of endoprosthesis in the early nineties, this noninvasive surgical modality has clearly gained popularity and is now acknowledged as the golden standard in the treatment of acute complicated tBADs. This has been shown by the IRAD-group already in 2008 (8). If the diagnosis of a complicated tBAD is confirmed, patients should be immediately brought to a hybrid operating room for endovascular and/or surgical treatment options. The goal of an endovascular treatment is to cover the entry tear and eventually the ruptured part of the aorta, depressurization of the false channel allowing full expansion of the true lumen and promoting thrombosis of the false lumen with subsequent aortic remodeling. In doing so, end organ ischemia can be reversed. Compared to open surgery, endografting is a minimal invasive and short intervention with reduced blood loss and a much faster recovery. Needless to say that thoracic endovascular aortic repair (TEVAR) has a reduced morbidity and mortality especially in complicated patients. Different treatment modalities are discussed.
Acute uncomplicated type B aortic dissection
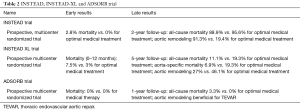
It is clear that medical treatment of acute uncomplicated tBAD is associated with a lower mortality compared to emergent open surgical treatment. Grosso modo 10% of the patients will survive their hospital stay with only optimal medical treatment. Overall, 89% of patients with acute uncomplicated tBAD survive to hospital discharge, although the in-hospital survival rates were as low as 29% for the highest risk group, 64% for the intermediate, and 97% for the lowest risk group (9,10). The problem however is that more than three quarters will develop future post dissection aneurysms requiring surgical treatment. Therefore, one can postulate that best medical treatment alone remains suboptimal. Not surprisingly elective TEVAR may play an important role in these particular patient-groups and to evaluate or clarify this, two industry sponsored trials have been conducted, the INSTEAD and the ADSORB trial. A summary of these trials is given in Table 2.
Full table
The INSTEAD (The INvestigation of STEnt Grafts in Aortic Dissection) trial (11) included uncomplicated tBAD patients in the subacute and chronic phase. Results showed a higher early mortality for the TEVAR-group compared to the optimal medical treatment group. The data in this trial show that elective stent-graft placement on top of optimized medical management fails to improve survival and adverse events within an observation period of 2 years despite favorable aortic remodeling (12). The extension of this trial, the INSTEAD-XL (13), showed that TEVAR did show favorable results between 2 and 5 years of follow-up: all-cause and aorta-specific mortality were improved with TEVAR in the long-term. Moreover, the TEVAR group showed less progression of dissection, suggesting remodeling of the aorta after 5 years, compared to the medically managed group (27.0% vs. 46.1%; P=0.04). TEVAR was also associated with stent graft-induced false lumen thrombosis in 90.6%, while the rate of false lumen thrombosis in patients treated with optimal medical treatment alone was 22.0% (P<0.001). Based on these results TEVAR combined with optimal medical therapy may emerge as the first line treatment for uncomplicated tBAD in patients with suitable anatomy.
The ADSORB trial (14) (acute dissection: stent graft OR Best medical therapy) is the first randomized trial on acute dissection and compares best medical treatment with best medical treatment plus thoracic stent grafting of the primary entry tear in patients with acute uncomplicated type B dissection. This multicenter, prospective randomized controlled trial, performed at 17 European centers, randomized 61 patients (31 best medical treatment group, 30 best medical treatment + TEVAR group), aged 18 to 80 years, with uncomplicated (no rupture, malperfusion, or refractory pain) acute tBAD (penetrating ulcer and intramural hematoma patients excluded), to best medical treatment or best medical treatment plus TEVAR. This trial was underpowered for survival, and had a cut-off at 1-year follow-up. Even though the follow-up was short, a benefit for TEVAR in terms of aortic remodeling was found. Incomplete false lumen thrombosis was seen in 43% of patients with TEVAR plus optimal medical treatment versus 97% in the optimal medical treatment group (P<0.001).
Despite very promising results of elective TEVAR in acute uncomplicated tBAD, it remains unclear if all uncomplicated patients without exception should nowadays be treated in this way. We might not forget that the number of reinterventions remain however substantial: over a median follow-up of 34 months, 26% of patients required a reintervention for various indications including endoleaks, distal fenestrations and metachronous pathology (15). So, the role of TEVAR in the management of the larger group of patients presenting with acute uncomplicated tBAD requires further delineation. Due to the lack of definitive clinical evidence, actually a patient-specific approach is currently advised in the acute uncomplicated tBAD taking into consideration certain risk factors. There is now scientific evidence showing predictors of outcome in acute tBAD such as the location of the primary entry tear. Patients with a location of the primary entry tear on the concavity of the distal arch and with a size of the entry tear larger than 10 mm, have more complicated dissections either at presentation or during hospitalization (16,17). Also, the size of the entry tear may be an important risk factor with regard to long-term prognosis of an initially uncomplicated tBAD. Evangelista et al. found that a large (≥10 mm) entry tear in the proximal part of the dissection leads to more rapid aortic expansion and a higher incidence of dissected-related events (18). Another very promising founding by Sato et al. illustrates the importance of morphological data of the shape of the true lumen as an effective predictive factor of aortic growth in tBAD (19). van Bogerijen and colleagues (20) have recently published a detailed review of the known predictors of aortic growth in uncomplicated tBAD. Sailer et al. (21) identified 5 significant predictors of adverse aortic events after uncomplicated tBAD: connective tissue disease, circumferential extent of the false lumen, the maximal aortic diameter, the false lumen outflow, and the number of intercostal arteries. By doing so the authors can stratify patients into low, intermediate and high risk of adverse events which may guide us in our decision making how to treat acute uncomplicated tBAD.
Acute complicated type B aortic dissection
Open surgery for acute, complicated tBAD is associated with an operative mortality in excess of 20%, sometimes much higher, and considerable morbidity including spinal cord ischaemia (22) and has actually almost disappeared. TEVAR for acute complicated tBAD is clearly superior to medical treatment or open surgery and is actually the golden standard in these sometimes dramatic circumstances (23). An observational study confirmed the beneficial outcomes of TEVAR for acute complicated tBAD with an in-hospital mortality of 4%, 40% and 33% for TEVAR, open surgery and medically treated patients respectively (24). Visceral malperfusion clearly still has a dramatic impact on outcome despite optimal patency of the visceral branches after TEVAR with a high 30-day mortality (25,26). In a systematic review and meta-analysis of complicated and uncomplicated acute tBAD, Moulakakis et al. found a significant lower 30-day mortality, incidence of stroke and spinal cord injury after TEVAR compared to open surgery (27).
Connective tissue disorders and TEVAR: yes or no?
Although feasible, the use of TEVAR in patients with connective tissue disorders such as Marfan, Ehlers-Danlos, and Loeys-Dietz syndromes remains contra-indicated because the aortic diameter will continue to dilate over time with higher reinterventions rates and higher risks for stent-graft related complications such as retrograde aortic dissection. Only in emergency situations TEVAR can be accepted in these particular patients as a bridge to definitive surgery (28,29). The future progression of the disease with unavoidable dilatation leads to secondary endoleaks and high reintervention rates with uncertain long-term results. For this reason, there is currently consensus that TEVAR should be limited to exceptional cases and emergency situations in patients with genetically linked aortic diseases (30).
Chronic type B aortic dissection
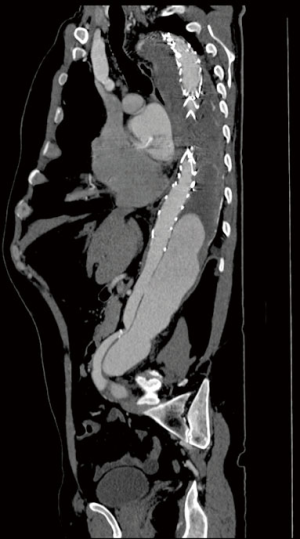
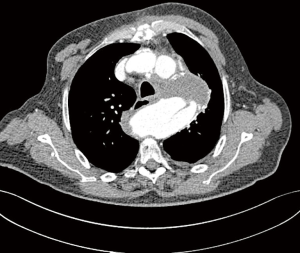
About 3 months after the acute onset of symptoms, patients reach a chronic stage and symptoms have disappeared. Most often the patient is followed by regular aortic imaging techniques and a gradual dilatation in the aortic diameter may become visible. The patient has developed a so-called chronic post dissection aortic aneurysm that can be limited to the descending aorta but very often extends below the diaphragm into the thoracoabdominal aorta. These extensive aneurysms are mostly asymptomatic, sometimes very large aneurysm may cause compression symptoms on surrounding organs such as oesophagus or bronchi. Indications for elective repair in asymptomatic patients include rapid aneurysm enlargement defined as >5-mm growth in 12 months or the absolute size. The interdisciplinary expert consensus document on the management of tBAD suggested to follow up uncomplicated patients with imaging surveillance and medical management while complicated cases can be treated by TEVAR or open surgery (31). For fusiform aneurysms, this includes a diameter of 6 cm (Figure 1) or more in non-connective tissue disorder patients or 5 cm in the latter category. There are de facto three treatment options: classic open surgery, total endovascular surgery or hybrid techniques. There are actually no randomized controlled trials comparing these three options.
The aim of open surgical repair is to replace the descending and/or thoracoabdominal aorta with a graft to restore peripheral and visceral perfusion, to exclude all dilated and dissected aortic tissue, and in doing so preventing aortic rupture. In my opinion surgery offers the best durable results with one definitive invasive treatment but it must be performed in centers with high aortic expertise. The risk of paraplegia has clearly been reduced over the last years by the introduction of some protective measures such as cerebrospinal fluid drainage, left heart bypass with permissive hypothermia or extracorporeal circulation with deep hypothermic circulatory, reimplantation of critical intercostals and/or lumbar arteries between T8 and L2, and intrathecal papaverine. In large aortic centers hospital mortality after open thoracoabdominal repair in elective cases is less than 10% with an incidence of paraplegia and acute renal failure less than 5% respectively (32). After open repair long-term survival and freedom from aortic re-intervention is excellent and should also be taken into account when evaluating lesser invasive alternatives.
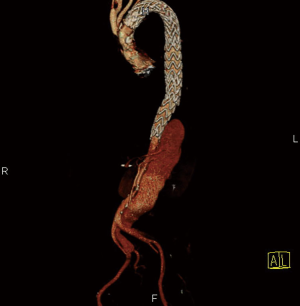
Despite the fact that endovascular treatment gains more and more popularity for these specific indications (chronic post dissection descending or thoracoabdominal aortic aneurysms), it is related to unsatisfactory results. I quote here Rohlffs et al. illustrating that the role of TEVAR is not well defined (33): “But the concept of TEVAR with implantation of a tubular stent-graft into the thoracic aorta to seal the proximal entry tear and reroute the blood flow into the true lumen alone, is not associated with satisfactory results. This is mainly due to the sparse remodeling capacity of the aortic tissue compared to earlier stages of the disease as the aortic wall and the dissection membrane are thickened and more rigid”. Indeed TEVAR for chronic tBAD fails because of the presence of uncovered distal fenestrations allowing for continued backfilling and pressurization of the false lumen. In addition, the thickened chronic intimal dissection flap may not immediately reapproximate to the native aortic wall and is thought to be less amenable to reverse remodeling. Midterm reintervention rate after TEVAR for chronic post dissection thoracoabdominal aortic aneurysms is 60% (34) and complete false lumen thrombosis is only 30% (35). Very often there is persisting false lumen flow in the lower 1/3 of the stented aorta (Figures 2,3). It is an option to cover just a limited thoracic aorta segment and observe the abdominal aorta by regular scans but it is the mainstay now to cover most of the thoracic aorta. This will certainly increase the risks of paraplegia. TEVAR for chronic tBAD limited to the descending thoracic aorta is technically feasible and is certainly associated with reduced procedural morbidity when compared with conventional open repair. As an adjunct one can use candy-plugs, knickerbocker grafts or simply occluder devices to close the false lumen and avoid retrograde filling into the descending aorta. Mid-term outcomes in properly selected patients are favorable, however long-term outcomes and the identification of patients who are prone to failure with endovascular therapy await further elucidation (36,37). Branched and fenestrated EVAR (BEVAR and FEVAR) offer certainly more opportunities in chronic tBAD of the thoracoabdominal aorta. However, these are very specialized endovascular techniques with a lot of pitfalls. Because the true lumen is very often narrow, the working space is limited making it technically very demanding. The visceral vessels may take off from the false or true lumen (in the ideal scenario all from the true lumen). The minimal acceptable proximal landing zone length is 2 cm proximal to the primary tear and can be a limiting factor. Very often there are multiple small abdominal fenestrations and after deployment of the main stent graft, target side branches may undergo rearrangement. Also, special imaging techniques such as fusion images are necessary. If we focus on the endovascular treatment of the complete thoracoabdominal aorta, the worldwide experience is very limited and results are not yet convincing. But it is clear that endovascular procedures continue to mature and in the future this BEVAR or FEVAR for chronic post dissection thoracoabdominal aortic aneurysms will be further improved and perfectionized allowing more patients to be treated by this very encouraging technique.
Hybrid techniques
A hybrid repair can be defined as a combined surgical and endovascular approach in which transperitoneal retrograde visceral revascularization is used to create an adequate distal landing zone for endovascular aneurysm exclusion. The endovascular stage can be performed during the debranching operation or later. The risk of the hybrid approach may be no lower than conventional open repair. There have been numerous case series evaluating the outcomes of hybrid TAA repairs. Thirty-day mortality can be as high as 30%. Chiesa et al. reported on 32 visceral patients with no intraoperative deaths but a perioperative mortality of 23% and morbidity of 30.8%. These authors concluded that hybrid TAAA repair did not lead to a significant improvement in outcomes compared with open TAAA repair in a similar group of patients (38). Biasi et al. (39) achieved a 16.7% early mortality in 18 patients with 7 early and late endoleaks. Long-term results are unknown but the hybrid option may be a viable alternative to open surgical repair in high-risk patients or in patients where anatomical constraints limit total endovascular repair.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Steuer J, Björck M, Mayer D, et al. Distinction between acute and chronic type B aortic dissection: is there a sub-acute phase? Eur J Vasc Endovasc Surg 2013;45:627-31. [Crossref] [PubMed]
- Booher AM, Isselbacher EM, Nienaber CA, et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med 2013;126:730.e19-24. [Crossref] [PubMed]
- Dake MD, Thompson M, van Sambeek M, et al. Dissect: a new mnemonic-based approach to the categorization of aortic dissection. Eur J VAsc Endovasc Surg 2013;46:175-90. [Crossref] [PubMed]
- Wheat MW Jr, Palmer RF, Bartley TD, et al. Treatment of dissecting aneurysms of the aorta without surgery. J Thorac Cardiovasc Surg 1965;50:364-73. [PubMed]
- Tran TP, Khoynezhad A. Current management of type B aortic dissection. Vasc Health Risk Manag 2009;5:53-63. [PubMed]
- Suzuki T, Eagle KA, Bossone E, et al. Medical management in type B aortic dissection. Ann Cardiothorac Surg 2014;3:413-7. [PubMed]
- DeBakey ME, Beall AC Jr, Cooley DA, et al. Dissecting aneurysms of the aorta. Surg Clin North Am 1966;46:1045-55. [Crossref] [PubMed]
- Fattori R, Tsai TT, Myrmel T, et al. Complicated Acute Type B Dissection: Is Surgery Still the Best Option? A Report From the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1:395-402. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Tsai TT, Fattori R, Trimarchi S, et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation 2006;114:2226-31. [Crossref] [PubMed]
- Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: The INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009;120:2519-28. [Crossref] [PubMed]
- Nienaber CA. Influence and critique of the INSTEAD Trial (TEVAR versus medical treatment for uncomplicated type B aortic dissection). Semin Vasc Surg 2011;24:167-71. [Crossref] [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [Crossref] [PubMed]
- Brunkwall J, Kasprzak P, Verhoeven E, et al. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg 2014;48:285-91. [Crossref] [PubMed]
- Hughes GC. Management of acute type B aortic dissection; ADSORB trial. J Thorac Cardiovasc Surg 2015;149:S158-62. [Crossref] [PubMed]
- Weiss G, Wolner I, Folkmann S, et al. The location of the primary entry tear in acute type B aortic dissection affects early outcome. Eur J Cardiothorac Surg 2012;42:571-6. [Crossref] [PubMed]
- Loewe C, Czerny M, Sodeck G, et al. A new mechanism by which an acute type B aortic dissection is primarily complicated, becomes uncomplicated, or remains uncomplicated. Ann Thorac Surg 2012;93:1215-22. [Crossref] [PubMed]
- Evangelista A, Salas A, Ribera A, et al. Long-term outcome of aortic dissection with patent false lumen: Predictive role of entry tear size and location. Circulation 2012;125:3133-41. [Crossref] [PubMed]
- Sato H, Ito T, Kuroda Y, et al. New predictor of aortic enlargement in uncomplicated type B aortic dissection based on elliptic Fourier analysis. Eur J Cardiothorac Surg 2017;52:1118-24. [Crossref] [PubMed]
- van Bogerijen GH, Tolenaar JL, Rampoldi V, et al. Predictors of aortic growth in uncomplicated type B aortic dissection. J Vasc Surg 2014;59:1134-43. [Crossref] [PubMed]
- Sailer AM, van Kuijk SM, Nelemans PJ, et al. Computed tomography imaging features in acute uncomplicated Stanford type-B aortic dissection predict late adverse events. Circ Cardiovasc Imaging 2017;10:e005709. [Crossref] [PubMed]
- Bozinovski J, Coselli JS. Outcomes and survival in surgical treatment of descending thoracic aorta with acute dissection. Ann Thorac Surg 2008;85:965-70; discussion 970-1. [Crossref] [PubMed]
- Zeeshan A, Woo EY, Bavaria JE, et al. Thoracic endovascular aortic repair for acute complicated type B aortic dissection: superiority relative to conventional open surgical and medical therapy. J Thorac Cardiovasc Surg 2010;140:S109-15. [Crossref] [PubMed]
- Jonker FH, Patel HJ, Upchurch GR, et al. Acute type B aortic dissection complicated by visceral ischemia. J Thorac Cardiovasc Surg 2015;149:1081-6.e1. [Crossref] [PubMed]
- Park WM, Gloviczki P, Cherry KJ Jr, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg 2002;35:445-52. [Crossref] [PubMed]
- Edwards MS, Cherr GS, Craven TE, et al. Acute occlusive mesenteric ischemia: Surgical management and outcomes. Ann Vasc Surg 2003;17:72-9. [Crossref] [PubMed]
- Moulakakis K, Mylonas S, Dalainas I, et al. Management of complicated and uncomplicated acute type B dissection. A systematic review and meta-analysis. Ann Cardiothorac Surg 2014;3:234-46. [PubMed]
- Waterman AL, Feezor RJ, Lee WA, et al. Endovascular treatment of acute and chronic aortic pathology in patients with Marfan syndrome. J Vasc Surg 2012;55:1234-40; disucssion 1240-1.
- Cooper DG, Walsh SR, Sadat U, et al. Treating the thoracic aorta in Marfan syndrome: Surgery or TEVAR? J Endovasc Ther 2009;16:60-70. [Crossref] [PubMed]
- Böckler D, Meisenbacher K, Peters AS, et al. Endovascular treatment of genetically linked aortic diseases. Gefasschirurgie 2017;22:1-7. [Crossref] [PubMed]
- Fattori R, Cao P, De Rango P, et al. Interdisciplinary Expert Consensus Document on Management of Type B Aortic Dissection. J Am Coll Cardiol 2013;61:1661-78. [Crossref] [PubMed]
- Alfonsi J, Murana G, Smeenk H, et al. Open surgical repair of post-dissection thoraco-abdominal aortic aneurysms: Early and late outcomes of a single-center study involving over 200 patients. Eur J Cardiothorac Surg. [Crossref] [PubMed]
- Rohlffs F, Tsilimparis N, Diener H, et al. Chronic type B aortic dissection: indications and strategies for treatment. J Cardiovasc Surg (Torino) 2015;56:231-8. [PubMed]
- Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632-47. [Crossref] [PubMed]
- Mani K, Clough RE, Lyons OT, et al. Predictors of Outcome after Endovascular Repair for Chronic Type B Dissection. Eur J Vasc Endovasc Surg 2012;43:386-91. [Crossref] [PubMed]
- Parsa CJ, Schroder JN, Daneshmand MA, et al. Midterm results for endovascular repair of complicated acute and chronic type B aortic dissection. Ann Thorac Surg 2010;89:97-102; discussion 102-4. [Crossref] [PubMed]
- Parsa CJ, Williams JB, Bhattacharya SD, et al. Midterm results with thoracic endovascular aortic repair for chronic type B aortic dissection with associated aneurysm. J Thorac Cardiovasc Surg 2011;141:322-7. [Crossref] [PubMed]
- Chiesa R, Tshomba Y, Melissano G, et al. Hybrid approach to thoracoabdominal aortic aneurysms in patients with prior aortic surgery. J Vasc Surg 2007;45:1128-35. [Crossref] [PubMed]
- Biasi L, Ali T, Loosemore T, et al. Hybrid repair of complex thoracoabdominal aortic aneurysms using applied endovascular strategies combined with visceral and renal revascularization. J Thorac Cardiovasc Surg 2009;138:1331-8. [Crossref] [PubMed]
Cite this article as: Schepens MA. Type B aortic dissection: new perspectives. J Vis Surg 2018;4:75.