How can genetic diagnosis inform the decision of when to operate?
Introduction
Aortic aneurysms—largely silent and potentially catastrophic—account for 1% to 2% of all deaths in the industrialized world (1). Clinicians have recognized two predominant spatial distributions of aortic aneurysms that vary considerably in risk factors, pathophysiology, and their natural and clinical histories (2): infrarenal abdominal aortic aneurysm (AAA) and thoracic aortic aneurysm (TAA). The development of infrarenal AAA is driven primarily by atherosclerosis and associated risk factors including age, smoking, hypertension, and male sex (3). In contrast, hereditary predisposition strongly influences the development of TAA, with a 10-fold increased risk in first-degree relatives (4,5). Genetically triggered aortopathies invariably involve the aortic root and ascending thoracic aorta, whereas no monogenic disorder has been described for isolated descending TAAs or AAAs. Clinical and genetic observations suggest that heritable thoracic aortic disease (HTAD) comprises single-gene disorders that are inherited in an autosomal dominant manner and primarily affect the proximal aorta.
Genetic discovery for HTAD has been progressing at a brisk pace, and the natural and clinical histories of each disorder and affected gene have begun to inform surgical management. Although a complete medical history, a detailed pedigree, and a comprehensive physical exam are still the cornerstones for establishing a diagnosis, genetic testing is useful for confirming the diagnosis, particularly for disorders that have considerable phenotypic overlap. Identifying a pathogenic variant in a known HTAD gene may be helpful in predicting how aortic disease will manifest and in estimating the risk of associated vascular diseases. Genetic testing in the affected proband also permits testing in asymptomatic family members to identify those individuals who need lifelong monitoring. Furthermore, in large families, more personalized pharmacological and surgical management may be achieved by combining genotypic data with a better understanding of genotype-phenotype correlations. In a recent review, Brownstein and colleagues of the Yale group (6) have presented an excellent summary of the 29 HTAD-associated genes identified to date.
Syndromic vs. non-syndromic TAAs
Patients with HTAD may be classified as having either a syndromic or non-syndromic disorder, with the latter encompassing familial thoracic aortic aneurysms (FTAAs) as well as TAA associated with bicuspid aortic valve (BAV) (Figure 1). The syndromes associated with syndromic aortic disorders include Marfan syndrome (MFS), Loeys-Dietz syndrome (LDS), and vascular Ehlers-Danlos syndrome (vEDS). These are multisystem genetic disorders that segregate in an autosomal-dominant manner. The extra-aortic manifestations typically affect the integumental, musculoskeletal, ocular, craniofacial, and cardiovascular systems. The phenotypic characteristics overlap between the disorders, but each has distinguishing characteristics and prognostic features.
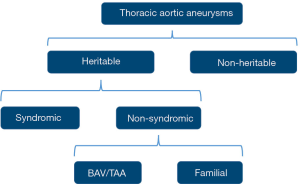
In non-syndromic TAAs, the abnormalities are limited to the cardiovascular system. The familial inheritance of TAAs was first identified in the late 1990s with the observation that up to 20% of individuals with non-syndromic thoracic aortic aneurysms and dissections (TAAD) have a positive family history (7,8). Moreover, this percentage is probably markedly underestimated as not all family members of patients with TAAD undergo routine imaging (9). FTAA is a heterogeneous group of non-syndromic TAA disorders with autosomal dominant inheritance, variable expression, and incomplete penetrance. Table 1 shows a list of genes associated with HTAD.

Full table
Marfan syndrome
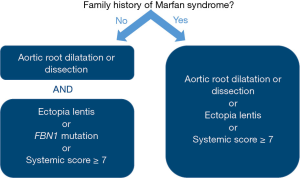
Initially described by a French pediatrician in 1896, MFS is characterized by manifestations in the eye (ectopia lentis and myopia), the skeletal system (overgrowth of long bones, arachnodactyly, scoliosis, and pectus deformities), and the cardiovascular system (aortic root aneurysm and mitral valve prolapse) (10). The cardiovascular manifestations are responsible for the excessive mortality and morbidity observed in these patients (11,12). In 2010, new diagnostic criteria for MFS—known as the revised Ghent nosology—were introduced that integrated genetic testing and gave more weight to the presence of aortic disease and ectopia lentis (Figure 2) (13). The online diagnostic tool at http://www.marfan.org/dx/home may aid in assessing patients. MFS is a monogenic disorder caused by mutations in FBN1, the gene that encodes the protein fibrillin-1 (14). In about 25% of probands, the disease arises de novo, but a clear family history is found in the remaining 75% of patients. To date, approximately 1,850 different mutations have been described (http://www.umd.be/FBN1/) of which the majority are unique to each family. Fibrillin-1 is an extracellular matrix glycoprotein that contributes to the formation of microfibrils, which are essential for the elasticity and structural support of numerous tissues.
Hence, FBN1 mutations were initially thought to lead to tissue fragility, exclusively through the disintegration and fragmentation of connective tissue fibers. However, the implication of over-activity of transforming growth factor-beta (TGF-β) signaling in the pathogenesis of MFS has changed the perception of fibrillin-1 from having just a structural role to having a role in dysregulated cell signaling (15,16).
In patients with MFS, the abnormality of the fibrous skeleton of the heart can lead to the development of aortic root aneurysms, as well as mitral valve prolapse (10). TAAs in MFS predominantly manifest as a pear-shaped dilatation of the aortic root, starting at the aortic annulus (annuloaortic ectasia) and extending into the proximal portion of the tubular ascending thoracic aorta. Elective root replacement before dissection develops has greatly improved the life expectancy of patients with MFS (11,17-19), although patients remain at risk for more distal aneurysms and dissections. Despite adequate medical management, 90% of patients with MFS will have aortic surgery or an aortic dissection in their lifetime, reflecting the lack of effective targeted therapies to prevent aortic events in these patients (10).
Loeys-Dietz syndrome and other TGF-β vasculopathies
The clinical features of LDS overlap substantially with those of MFS, and it was not recognized as a separate disease until 2005 (20). Characteristic features of LDS include hypertelorism, craniosynostosis, bifid uvula, cleft palate, and arterial tortuosity as well as TAAD. Patients with LDS may also have systemic manifestations of MFS (pectus deformity, scoliosis, and arachnodactyly) or EDS (easy bruising, thin skin, and uterine rupture with pregnancy). Compared with MFS, LDS has more severe and disseminated cardiovascular manifestations; dissections and ruptures occur at smaller aortic diameters, in peripheral arterial beds, and at younger ages (21,22). TAAs in patients with LDS can increase in diameter more than 1.0 cm per year, and one-third of patients experience a vascular event (dissection, surgery, or death from aortic dissection or rupture) before 19 years of age (23,24). Arterial tortuosity is typically observed in the neck vessels and is a known marker of adverse aortic outcomes (25). Ectopia lentis is not a feature of LDS.
Mutations in genes encoding TGF-β receptor I (TGFBR1) and receptor II (TGFBR2) were the first reported genetic causes of LDS (20,23). Subsequently, gene mutations in other components of the TGF-β signaling pathway, including SMAD3, and the TGF-β2 ligand genes 2 (TGFB2) and 3 (TGFB3), have been identified as causes of HTAD (21,26-32). Although initially identified in patients with syndromic presentations, mutations in TGF-β pathway genes have also been identified in isolated FTAA (33,34). It is increasingly recognized that patients and families with TGF-β vasculopathies present with a phenotypic continuum of various clinical features ranging from mild to severe extra-aortic manifestations (35). However, it is unclear why some mutations cause LDS and others result in mild disease. Detailed genotypic and phenotypic correlations have not emerged, but preliminary findings suggest that families with TGFB2/3 mutations have a less severe cardiovascular phenotype and a higher degree of non-penetrance than those with TGFBR1/2 mutations (36,37).
Smooth muscle contraction vasculopathies
Vascular smooth muscle cells (SMCs), which are the basic contractile unit of the aorta, regulate pressure and flow. The SMC contractile apparatus contributes to aortic structure and function, and mutations in these molecules play an important role in TAAD pathogenesis. Causative mutations associated with FTAA have been identified in genes encoding smooth muscle-specific alpha-actin (ACTA2), vascular smooth muscle contractile protein beta-MHC (MYH11), myosin light chain kinase (MYLK), and a type 1 cyclic guanosine monophosphate-dependent protein kinase that controls SMC relaxation (PRKG1). Heterozygous mutations of ACTA2 interfere with actin filament assembly and account for 10% to 14% of all FTAAs (38). Patients present with TAA, livedo reticularis, iris flocculi, patent ductus arteriosus, and non-thoracic aneurysms. These patients may also develop occlusive disease as a result of SMC hypertrophy, including premature coronary artery disease, ischemic stroke, and Moyamoya disease. MYH11 encodes SMC myosin heavy chain, which is a major contractile protein in vascular smooth muscle. Mutations in MYH11 are found in patients with FTAA and are associated with patent ductus arteriosus (39). Mutations in MYLK account for 1% of patients with FTAA and are associated with acute aortic dissection with little or no aortic enlargement (40).
Bicuspid aortic valve associated thoracic aneurysms
The prevalence of dilatation of the ascending aorta among patients with BAV ranges from 20% to 84% (41). Different patterns of BAV aortopathy have been identified; some primarily affect the ascending aorta with or without the proximal arch, and others predominantly involve the aortic root, often with annuloaortic ectasia. Of note, family members of patients with BAV-associated aortopathy may have BAV and TAAD, TAAD with a tricuspid aortic valve, or BAV without TAAD (42). First-degree relatives of patients with BAV should be screened for both valvular abnormalities and TAAD.
Although proximal aortic aneurysms are common in patients with BAV, the incidence of aortic dissection in contemporary series has been lower than previously suspected. In a Toronto study of 642 patients with BAV, the incidence of aortic dissection was 0.1% per patient year of follow-up (43). In a report from Olmstead County, patients with BAV were 86 times more likely to have proximal aortic aneurysms and 8 times more likely to experience aortic dissection compared to controls (44). Despite this high relative risk of dissection, the absolute risk was quite low at 3.1 cases per 10,000 person-years during a mean follow-up of 16±7 years. Furthermore, no dissections were reported in patients with an aortic diameter smaller than 4.5 cm at baseline or in those with a normally functioning aortic valve (44). Multispecialty guidelines have increased the threshold for elective replacement of the proximal aorta from 5.0 to 5.5 cm in patients with BAV who have no additional risk factors (45-47). Emerging literature also supports a more conservative approach to the aortic root in these patients. For example, recent reports suggest that the remaining sinus segments do not dilate at a clinically meaningful rate after replacement of the ascending aorta in patients with BAV who do not have an aortic root aneurysm at the time of operation (48,49). Thus, a prophylactic root replacement procedure may not be warranted in patients with non-dilated sinus segments at the time of ascending aortic surgery.
Surgical management of HTAD: when and how?
Surgical thresholds
The surgical threshold for elective replacement of the proximal aorta in patients without HTAD is based on the few classic clinical history studies conducted by the Yale group (50,51). Although diameter is the best-known predictor of catastrophic aortic dissections, aortic dissections—including DeBakey types I and II (Stanford type A) and DeBakey type III (Stanford type B)—commonly occur at aortic dimensions not considered aneurysmal (52,53). Factors other than absolute size should be considered when deciding whether or not to recommend elective aneurysm repair (Table 2). As patients with thoracic aortic aneurysms are most often asymptomatic, surgery is generally suggested only to those in whom the risk of rupture or dissection is considered significant in the context of their overall life expectancy.

Full table
Patients with HTAD who have either a known genetic cause or a family history of TAAD should undergo elective repair of their aorta at smaller diameters than those with non-heritable aneurysms. As our knowledge of the clinical history of patients with HTAD evolves, gene-specific and even mutation-specific recommendations may be possible. Figure 3 summarizes our current recommendations regarding surgical thresholds for asymptomatic proximal aortic aneurysms in patients with HTAD. In deciding on the exact diameter at which to recommend aortic repair, physicians should weigh factors that affect the individual’s risk of aortic catastrophe against the perioperative risks of the proposed intervention. For example, for a patient with a TGFBR1 mutation without either syndromic features or a malignant family history, it would be reasonable to wait until a threshold of 4.5 cm is reached before recommending elective root repair; however, for a patient with a TGFBR1 mutation, a high degree of arterial tortuosity, and a family history of aortic dissection at small dimensions, using a lower threshold and recommending root repair at a diameter of 4.0 cm would be prudent. Similarly, for those with a TGFBR2 mutation, a threshold of 4.5 cm would be reasonable in a man with mild phenotypic features, whereas a threshold of 4.0 cm would be more appropriate in a woman who has a small body surface area or severe phenotypic features, such as wide scars (35). When interpreting aortic measurements and expansion of the aorta, it is important to consider the variability of diameter measurements; for example, computed tomography measurements of proximal aortic diameter can vary between 1.6 to 5 mm (54). Thus, apparent small changes in the diameter on serial imaging studies, especially non-electrocardiographically gated studies, may be within the measurement error. This may be particularly important when evaluating serial studies for patients with HTAD and genetic variants for whom repair has been recommended at lower diameters (4.0–4.5 cm).
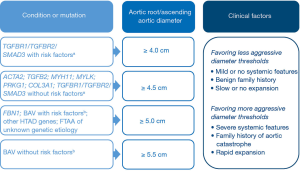
A tailored approach based on clinical factors is currently recommended for patients with BAV (47). For those who are undergoing surgery to address aortic valve dysfunction, concomitant replacement of the ascending aorta is recommended if the aortic diameter is ≥4.5 cm. For those who do not have a valvular indication for surgery, replacement of the aortic root and/or ascending aorta is recommended if the aortic diameter is ≥5.0 cm for BAV patients with rapid aortic dilatation (>0.5 cm per year) or a family history of aortic dissection, and if the diameter is ≥5.5 cm for patients without additional aortic risk factors.
The data are insufficient to recommend gene-specific thresholds for elective repair of the aortic arch and thoracoabdominal aorta in patients with HTAD. Total arch and thoracoabdominal aortic repairs are associated with higher perioperative morbidity and mortality than elective root repair, and as such the recommended dimensions for repair are larger for the distal aorta than for the proximal aorta. Patients with HTAD, particularly those with a chronic dissection, should undergo repair of the distal aorta at dimensions smaller than those recommended for patients with non-heritable aneurysms. Ultimately, surgeons must balance the risk of the proposed intervention against the risk of aortic catastrophe when evaluating patients with TAAD.
Special populations
During pregnancy, hemodynamic alterations and changes in the aortic media lead to an increased rate of aortic dilatation. The incidence of aortic dissection is estimated to be 100-fold higher in the general population during pregnancy, and the risk is likely greater in patients with HTAD (55). Women contemplating pregnancy who are at risk of aortic events should undergo a preconception evaluation that includes baseline cross-sectional imaging and risk stratification in a high-risk pregnancy clinic. The option of in-vitro fertilization with pre-implantation genetic diagnosis should be discussed. Table 3 summarizes our suggested thresholds for elective pre-pregnancy aortic root repair. Elective root replacement does not guarantee the absence of risk, as the distal aorta remains susceptible to acute dissection. Close imaging surveillance with echocardiography or magnetic resonance imaging is recommended during pregnancy and the post-partum period; beta-blockers and blood pressure control are the mainstay of medical therapy for those at risk of rupture and dissection (56). Pregnant women with HTAD should have a clear plan for labor and delivery formulated by a multidisciplinary team of obstetricians, cardiologists, and cardiovascular surgeons.

Full table
Technical considerations
We advocate for more aggressive aortic resection in patients with known HTAD to reduce the likelihood of reintervention on adjacent segments of aorta. For example, patients with aortic root aneurysm who have HTAD often have annuloaortic ectasia, and stabilizing the annulus at the time of root replacement would be beneficial for these patients. In patients with MFS, valve-sparing root replacement with a reimplantation technique has been shown to be a more durable strategy than remodeling (18). Although the literature is sparse, we believe similar results would be observed for patients with LDS and other genetic conditions associated with annuloaortic ectasia. We argue against partial root replacement (i.e., replacement of only one or two of the sinuses) in those with HTAD and an aortic root aneurysm. With regard to the distal aorta, we do not have data to support prophylactically replacing the arch during proximal aortic surgery in patients with a non-dilated arch. It is reasonable, however, to replace the proximal arch (hemi-arch repair) in patients with HTAD during proximal aortic repair if it can be achieved with limited incremental risk (57). During replacement of the transverse arch or the thoracoabdominal aorta, we advocate for the use of individual branched grafts to the supra-aortic and visceral vessels, respectively. The use of large Carrel patches (islands) for patients with HTAD is not advised as patch aneurysms may develop in the remnant segments of the aorta.
The role of endovascular stent grafts in patients with HTAD has been limited and requires further study. In patients with MFS treated with thoracic endovascular aortic repair (TEVAR), high rates of stent migration and endoleaks and even cases of aortic rupture have been reported (58-61). Endovascular stent-grafts have been successfully used as a life-saving bridge to definitive repair (62) and in cases where the stent-graft is anchored in Dacron proximally and distally (63). In light of the poor outcomes of standard TEVAR procedures in patients with HTAD, we recommend restricting the use of such procedures to patients who are truly not candidates for open repair. Future developments in technology may further refine and expand the currently limited role of endovascular aortic repair for the management of patients with HTAD.
Conclusions
Although evolving medical and surgical approaches have significantly improved the life expectancy of patients with HTAD, additional strategies to further decrease aortic events are warranted. Surgical management of TAAD has become more personalized, with genetic factors increasingly informing the decision of when to operate on patients. An improved understanding of genotype-phenotype correlations in patients with HTAD will ultimately lead to gene- and mutation-specific recommendations for surgical repair. Until more robust data from larger cohorts can inform our decisions, patients with HTAD should be seen by an aortic specialist to develop a tailored approach to elective surgical repair.
Acknowledgements
The authors thank Rebecca Bartow, PhD, Senior Scientific Editor/Writer in the Scientific Publications Department at the Texas Heart Institute, for her editorial assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011;473:308-16. [Crossref] [PubMed]
- Pyeritz RE. Marfan syndrome: 30 years of research equals 30 years of additional life expectancy. Heart 2009;95:173-5. [Crossref] [PubMed]
- Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med 2009;361:1114-6. [Crossref] [PubMed]
- Isselbacher EM, Lino Cardenas CL, Lindsay ME. Hereditary influence in thoracic aortic aneurysm and dissection. Circulation 2016;133:2516-28. [Crossref] [PubMed]
- Albornoz G, Coady MA, Roberts M, et al. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg 2006;82:1400-5. [Crossref] [PubMed]
- Brownstein AJ, Ziganshin BA, Kuivaniemi H, et al. Genes associated with thoracic aortic aneurysm and dissection: an update and clinical implications. Aorta (Stamford) 2017;5:11-20. [Crossref] [PubMed]
- Biddinger A, Rocklin M, Coselli J, et al. Familial thoracic aortic dilatations and dissections: a case control study. J Vasc Surg 1997;25:506-11. [Crossref] [PubMed]
- Coady MA, Davies RR, Roberts M, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg 1999;134:361-7. [Crossref] [PubMed]
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010;55:841-57. [Crossref] [PubMed]
- Pyeritz RE. Recent progress in understanding the natural and clinical histories of the Marfan syndrome. Trends Cardiovasc Med 2016;26:423-8. [Crossref] [PubMed]
- Murdoch JL, Walker BA, Halpern BL, et al. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med 1972;286:804-8. [Crossref] [PubMed]
- Cook JR, Carta L, Galatioto J, et al. Cardiovascular manifestations in Marfan syndrome and related diseases; multiple genes causing similar phenotypes. Clin Genet 2015;87:11-20. [Crossref] [PubMed]
- Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476-85. [Crossref] [PubMed]
- Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991;352:337-9. [Crossref] [PubMed]
- Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 2003;33:407-11. [Crossref] [PubMed]
- Ramachandra CJ, Mehta A, Guo KW, et al. Molecular pathogenesis of Marfan syndrome. Int J Cardiol 2015;187:585-91. [Crossref] [PubMed]
- Gott VL, Greene PS, Alejo DE, et al. Replacement of the aortic root in patients with Marfan's syndrome. N Engl J Med 1999;340:1307-13. [Crossref] [PubMed]
- David TE, David CM, Manlhiot C, et al. Outcomes of Aortic Valve-Sparing Operations in Marfan Syndrome. J Am Coll Cardiol 2015;66:1445-53. [Crossref] [PubMed]
- Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. Am J Cardiol 1995;75:157-60. [Crossref] [PubMed]
- Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37:275-81. [Crossref] [PubMed]
- MacCarrick G, Loeys B, Dietz HC 3rd. Response to Pyeritz et al. Genet Med 2014;16:642-4. [Crossref] [PubMed]
- Williams JA, Loeys BL, Nwakanma LU, et al. Early surgical experience with Loeys-Dietz: a new syndrome of aggressive thoracic aortic aneurysm disease. Ann Thorac Surg 2007;83:S757-63; discussion S85-90.
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788-98. [Crossref] [PubMed]
- Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg 2012;56:565-71. [Crossref] [PubMed]
- Morris SA, Orbach DB, Geva T, et al. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation 2011;124:388-96. [Crossref] [PubMed]
- van de Laar IM, Oldenburg RA, Pals G, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet 2011;43:121-6. [Crossref] [PubMed]
- Regalado ES, Guo DC, Villamizar C, et al. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res 2011;109:680-6. [Crossref] [PubMed]
- Lindsay ME, Schepers D, Bolar NA, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 2012;44:922-7. [Crossref] [PubMed]
- Rienhoff HY Jr, Yeo CY, Morissette R, et al. A mutation in TGFB3 associated with a syndrome of low muscle mass, growth retardation, distal arthrogryposis and clinical features overlapping with Marfan and Loeys-Dietz syndrome. Am J Med Genet A 2013;161A:2040-6. [Crossref] [PubMed]
- Bertoli-Avella AM, Gillis E, Morisaki H, et al. Mutations in a TGF-beta ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol 2015;65:1324-36. [Crossref] [PubMed]
- Jondeau G, Boileau C. Familial thoracic aortic aneurysms. Curr Opin Cardiol 2014;29:492-8. [Crossref] [PubMed]
- Pyeritz R, Jondeau G, Moran R, et al. Loeys-Dietz syndrome is a specific phenotype and not a concomitant of any mutation in a gene involved in TGF-beta signaling. Genet Med 2014;16:641-2. [Crossref] [PubMed]
- Pannu H, Fadulu VT, Chang J, et al. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation 2005;112:513-20. [Crossref] [PubMed]
- Tran-Fadulu V, Pannu H, Kim DH, et al. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J Med Genet 2009;46:607-13. [Crossref] [PubMed]
- Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: Results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet 2016;9:548-58. [Crossref] [PubMed]
- Ritelli M, Chiarelli N, Dordoni C, et al. Further delineation of Loeys-Dietz syndrome type 4 in a family with mild vascular involvement and a TGFB2 splicing mutation. BMC Med Genet 2014;15:91. [Crossref] [PubMed]
- Kuechler A, Altmuller J, Nurnberg P, et al. Exome sequencing identifies a novel heterozygous TGFB3 mutation in a disorder overlapping with Marfan and Loeys-Dietz syndrome. Mol Cell Probes 2015;29:330-4. [Crossref] [PubMed]
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007;39:1488-93. [Crossref] [PubMed]
- Zhu L, Vranckx R, Khau Van Kien P, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 2006;38:343-9. [Crossref] [PubMed]
- Wang L, Guo DC, Cao J, et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet 2010;87:701-7. [Crossref] [PubMed]
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014;370:1920-9. [Crossref] [PubMed]
- Loscalzo ML, Goh DL, Loeys B, et al. Familial thoracic aortic dilation and bicommissural aortic valve: a prospective analysis of natural history and inheritance. Am J Med Genet A 2007;143A:1960-7. [Crossref] [PubMed]
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317-25. [Crossref] [PubMed]
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104-12. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-e129. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2016;67:724-31. [Crossref] [PubMed]
- Milewski RK, Habertheuer A, Bavaria JE, et al. Fate of remnant sinuses of Valsalva in patients with bicuspid and trileaflet valves undergoing aortic valve, ascending aorta, and aortic arch replacement. J Thorac Cardiovasc Surg 2017;154:421-32. [Crossref] [PubMed]
- Peterss S, Bhandari R, Rizzo JA, et al. The Aortic Root: Natural History After Root-Sparing Ascending Replacement in Nonsyndromic Aneurysmal Patients. Ann Thorac Surg 2017;103:828-33. [Crossref] [PubMed]
- Coady MA, Rizzo JA, Goldstein LJ, et al. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin 1999;17:615-35. vii. [Crossref] [PubMed]
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27; discussion 27-8. [Crossref] [PubMed]
- Pape LA, Tsai TT, Isselbacher EM, et al. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007;116:1120-7. [Crossref] [PubMed]
- Trimarchi S, Jonker FH, Hutchison S, et al. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J Thorac Cardiovasc Surg 2011;142:e101-7. [Crossref] [PubMed]
- Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2015;28:119-82. [Crossref] [PubMed]
- Wanga S, Silversides C, Dore A, et al. Pregnancy and Thoracic Aortic Disease: Managing the Risks. Can J Cardiol 2016;32:78-85. [Crossref] [PubMed]
- Bons LR, Roos-Hesselink JW. Aortic disease and pregnancy. Curr Opin Cardiol 2016;31:611-7. [Crossref] [PubMed]
- Malaisrie SC, Duncan BF, Mehta CK, et al. The addition of hemiarch replacement to aortic root surgery does not affect safety. J Thorac Cardiovasc Surg 2015;150:118-24. e2.
- Dong ZH, Fu WG, Wang YQ, et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation 2009;119:735-41. [Crossref] [PubMed]
- Ehrlich MP, Nienaber CA, Rousseau H, et al. Short-term conversion to open surgery after endovascular stent-grafting of the thoracic aorta: the Talent thoracic registry. J Thorac Cardiovasc Surg 2008;135:1322-6. [Crossref] [PubMed]
- Nordon IM, Hinchliffe RJ, Holt PJ, et al. Endovascular management of chronic aortic dissection in patients with Marfan syndrome. J Vasc Surg 2009;50:987-91. [Crossref] [PubMed]
- Waterman AL, Feezor RJ, Lee WA, et al. Endovascular treatment of acute and chronic aortic pathology in patients with Marfan syndrome. J Vasc Surg 2012;55:1234-40; discussion 40-1. [Crossref] [PubMed]
- Matos JM, de la Cruz KI, Ouzounian M, et al. Endovascular repair as a bridge to surgical repair of an aortobronchial fistula complicating chronic residual aortic dissection. Tex Heart Inst J 2014;41:198-202. [Crossref] [PubMed]
- Cooper DG, Walsh SR, Sadat U, et al. Treating the thoracic aorta in Marfan syndrome: surgery or TEVAR? J Endovasc Ther 2009;16:60-70. [Crossref] [PubMed]
Cite this article as: Ouzounian M, LeMaire SA. How can genetic diagnosis inform the decision of when to operate? J Vis Surg 2018;4:68.


