Pancreaticoduodenectomy: minimizing the learning curve
Introduction
Pancreatic resection is a complex undertaking that can be associated with significant patient morbidity. This operation was first introduced in the United States in the early 1930s by Whipple et al. (1), who himself performed only 37 operations in his career with a mortality rate of approximately 33% (2). Since then, the surgical community as a whole has undergone a very prolonged learning curve over multiple decades. Over the past 20 years, however, the operation has become a relatively common procedure with low mortality and reasonable morbidity. Regionalization and centralization of this particular procedure promises a reduction of postoperative morbidity and mortality and concentrates operative experience among surgeons and multidisciplinary teams that optimize supportive care before, during, and after the operation. A recent study of Medicare patients revealed that there is an ongoing shift towards high-volume hospitals, which has resulted in a decreased mortality rate for patients undergoing proximal pancreatectomy (3). Specifically regarding operative mortality after pancreaticoduodenectomy (PD), multiple studies have shown a clear association between hospital volume and outcomes (4-6). Additionally, several high-volume, single-institutional studies have indicated that there is an additional benefit from higher individual surgeon volumes for PD outcomes (7-10).
While the reasons for improved results seen over the past several decades are multifactorial, outcomes of surgical patients are still heavily influenced by the manner in which they are performed inside the operating room. An individual surgeon’s outcomes will improve as they progress from competency to subsequent proficiency and mastery of a particular procedure. The subject of a learning curve for a surgeon performing PD is frequently discussed, but objective data is seldom reported. Several publications have indicated that, over time, there are significant improvements in factors such as blood loss, operative time, complications, length of stay (LOS), readmissions, and margin negative resections among other factors for surgeons performing PD at the beginning of their careers (7,11-13). The concept of a surgeon’s learning curve can widely vary based on number of cases over a variable time period of several years to even a decade. Generally, it has been accepted that most surgeons will obtain proficiency at around 60 PDs, but improvements will continue to occur over the course of a surgeon’s career (7). Despite the continued need for learning new skills, the professional and public tolerance for a learning curve is much less than in previous times.
In the past decade, pancreatic surgical training in the United States has been greatly improved through standardization and an oversight process carried out by the America Hepato-Pancreato-Biliary Association through hepato-pancreato-biliary (HPB) fellowship training, where competency is measured by a case volume metric to measure experience (14). Ideally, in the setting of advanced dedicated HPB training, competency would be achieved in fellowship and proficiency obtained early within the surgeon’s career to avoid subjecting patients to the early learning curve outcomes, representing the natural evolution of surgical progress from previous eras. However, very little specific information is given regarding the methodology of surgical training or data regarding the outcomes of those surgeons beginning their career under current training conditions. In particular, the utility of minimally invasive surgical techniques in HPB surgery is rapidly increasing and adds a layer of complexity to training. Nonetheless, it may be possible that there is not only subsequent patient benefit from minimally invasive techniques, but also benefit to those undergoing pancreatic surgical training. Laparoscopic PD (LPD) has been shown to be a safe alternative to open PD (OPD) (15). From an education stand point, the approach has the advantage of an unrestricted surgical view of the entire operative field to those participating or observing the operation. It also allows for a much better understanding of the anatomic relationship between the pancreas and surrounding structures. Furthermore, since the procedure is easily recorded, it permits the learner to retrospectively review the video of the operation on a regular basis. We theorize that, in this manner, experienced surgeons can pass surgical technique to trainees in a more fluid and effective manner than ever before. The aim of this study was to describe the training method and retrospectively review the initial outcomes and subsequent learning curve of a pancreatic surgeon performing PD.
Methods
Information was prospectively collected on all patients undergoing pancreatic resection from July 2011 to June 2015 and retained in an institutional review board-approved database. Data points collected included demographics, operative variables, postoperative outcomes, pathologic findings, and extended follow-up. Preoperative characteristics included age, sex, co-morbidities, body mass index, American Society of Anesthesiologists score, Eastern Cooperative Oncology Group score, and use of neoadjuvant treatments. Operative details included operative time (incision to closure of the wound), estimated blood loss (EBL), and blood product transfusion obtained from the anesthesia record. Use of laparoscopy, type of PD (Whipple versus total pancreatectomy), vein resection, and concomitant resections were recorded in the database. Postoperative outcomes were tracked for 3 months (90 days) after surgery and graded according to the Clavien system (16). Final overall patient complication grade was given to the highest-rated complication grade experienced by the patients in the group. Minor complications included grades I and II, while major complications included grades III–V. Pancreatic fistula (PF) (17), post-pancreatectomy hemorrhage (18), and delayed gastric emptying (19) were scored and graded according to standard international consensus definitions. LOS was recorded and did not include the day of the operation, but included the day of discharge, while readmission was tracked for all patients to any hospital for 90 days after surgery. Reoperation and readmission were defined as any unplanned operation or admission, respectively, within 90 days of the primary procedure related to the pancreatic resection. Final pathologic details were recorded, as well as margin status and lymph node harvest.
Patients included in the study were those patients undergoing consecutive PD for curative intent during the study time period by the senior author (JA Stauffer). Those patients undergoing other pancreatic operations, such as distal pancreatectomy, central pancreatectomy, enucleation, pancreatic necrosectomy, drainage procedures, or palliative procedures, were excluded from this analysis to allow for a more homogenous study.
The first author completed a 1-year minimally invasive advanced gastrointestinal (GI) fellowship accredited by the Fellowship Council that ended in June 2011 and began to practice the next month, which was also when the study commenced. The author’s 5-year general surgical training was spent at the author’s current institution and included a substantial exposure to open hepatobiliary and liver transplantation operations.
This study encompasses all patients undergoing the included procedures during the primary author’s first 48 months of independent practice as an attending within a high-volume practice and institution. Patients were evaluated for appropriateness for resection in an outpatient clinic. Commonly, patients considered for resection were presented at a weekly multidisciplinary pancreatic pathology board with subspecialties, including radiology, gastroenterology, medical and radiation oncology, and senior surgeons. Decisions regarding patient management were confirmed in this fashion.
Operative technique
PD, laparoscopic and open, were performed in the same manner as previously described (15). The basic principle of the resection technique involves avoiding large morbid incisions by performing LPD through a 6-trocars or OPD through a small upper midline incision (12–15 cm), meticulous and bloodless dissection using natural anatomic planes, wide exposure with dissection done under direct visualization at all times, and peripancreatic dissection that allows for extensive exposure of the vasculature and generous lymphadenectomy. A pylorus preserving resection was preferred, but a standard (hemi-gastrectomy) resection was utilized as dictated by tumor involvement or previous gastric surgery.
Reconstruction was performed by duct to mucosa pancreaticojejunostomy with an inner layer of interrupted 5-0 absorbable braided sutures and outer layer of running 4-0 non-absorbable monofilament sutures. Internalized pancreatic duct stenting was routine in the first half of the study, but rarely used in the second half. Biliary reconstruction was performed in a single layer fashion with a running absorbable braided suture. Reconstruction for both LPD and OPD proceeded in the same manner, including order (biliary, pancreatic, and then gastrointestinal), suture/needle selection, and careful attention to a watertight mucosa to mucosa anastomosis under excellent visualization.
A single drain was placed behind the hepaticojejunostomy and pancreaticojejunostomy in all cases. Two drains were used if the patient was considered a high risk for PF. Total pancreatectomy was performed in a similar fashion, with the addition of splenic resection and absence of pancreatic reconstruction.
Learning curve analysis
Patients were divided into four groups based on the date of surgery. Each period included all patients undergoing PD over a 12-month time frame based on when the study began. All data collected during these time periods (year 1–4) were analyzed for trends of significance. Specific attention was paid to trends in morbidity rate, PF rates, operative time, EBL, LOS, and readmission rates over the 48-month study that represented the author’s learning curve of PD.
Statistical analysis
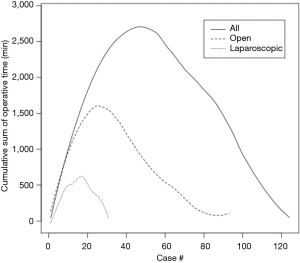
Descriptive statistics for categorical variables were reported as frequency and percentage; continuous variables were reported as median with range. Trends of change for categorical variables were tested using Cochran-Armitage trend test, and continuous variables were tested using Pearson correlation test. Cumulative sum analysis was used to illustrate the learning curve throughout the study by graphically depicting the cumulative sum differences from the total cohort’s mean. All tests were two-sided, with alpha level set at 0.05 for statistical significance.
Results
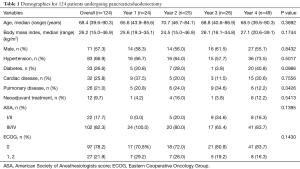
In the study time period, 124 patients underwent PD with 24, 25, 26, and 49 patients undergoing their operations in year 1, 2, 3, and 4, respectively. Patient demographics are given in Table 1. There was little variability over the course of the study. Thirty patients (24.2%) were ≥75 years of age, and the majority had a cardiovascular or pulmonary comorbidity. Thirty-three patients (26.7%) were noted to be obese with a body mass index ≥30 kg/m2. Operative variables are found in Table 2. Thirty-one patients (25.0%) underwent a laparoscopic procedure. Conversion from LPD to OPD was required in five patients (16.1%). Seventeen (13.7%) underwent a total pancreatectomy overall, the use of which significantly increased over time. Operative time statistically improved over the course of the study period. Cumulative sum analysis (Figure 1) shows that there was a learning curve of approximately 50 cases (30 open, 20 laparoscopic) in which operative time improved. EBL and number of patients requiring a blood transfusion remained stable over the time periods. Half of the patients in the series undergoing vein resection did so in year 4 (n=4). Over half undergoing an additional procedure underwent this in year 4, including hepatectomy (n=3), extended biliary resection (n=1), nephrectomy (n=1), colectomy (n=1), extensive ventral hernia repair (n=1), hiatal hernia repair (n=1), and gastric band removal (n=1).
Full table

Full table
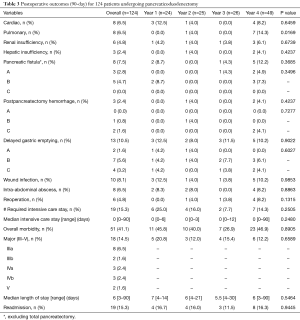
Postoperative complications are given in Table 3. Overall and clinically relevant morbidity was found in 41.1% and 14.5% of patients, respectively.
Full table
Clinically relevant PF was found in five patients (4.7%) undergoing a Whipple with no instances of grade C fistula. There was no significant variability in complications, LOS, or readmission rate over the time period except for an increase in pulmonary complications seen in the last period of the study. Median LOS was 6 days with 119 patients (96.0%) discharged home from the hospital. Use of intensive care and readmission, both seen at a rate of 15% of the population, was infrequent over the study time period. There were 2 (1.6%) mortalities seen in this cohort in a 90-day postoperative time period.
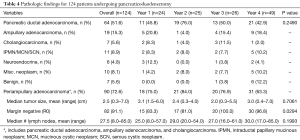
Pathology analysis is given in Table 4. The large majority of patients underwent pancreatic resection for neoplastic disease, mainly pancreatic ductal adenocarcinoma (n=64, 51.6%). For those patients with periampullary adenocarcinoma (n=90, 72.6%), the size of the primary tumor and number of lymph nodes harvested did not significantly vary over the time period. A negative margin was obtained in 91.1% of cases which significantly improved from the first half of the study to the second half.
Full table
Discussion
The treatment of patients with disease in the head of the pancreas, from clinical decision making to postoperative recovery, is complicated and best performed by an experienced multidisciplinary team. The surgeon’s role in executing a safe and effective PD is a key driver in the success of the overall care. Because of this, there has been significant emphasis on factors that improve the quality of its performance. One of these factors is how to properly train and monitor those surgeons who are doing these operations at the beginning of their career. It has been accepted that a learning curve exists for these surgeons, but little has been published regarding the details of this learning curve, or more importantly, how to avoid a learning curve that impacts patient outcomes. Given today’s focus on quality outcomes, the professional and public tolerance for subpar outcomes due to a learning progression is much less than in previous decades. Therefore, this study was performed to report the methods of training, mentoring, supervision, and assimilation of a pancreatic surgeon into a high-volume pancreatic practice, minimizing or avoiding a learning curve. Data was collected and monitored in a prospective fashion in order to safely perform this study. Outcomes, including complications, readmission, and mortality, were carried out to 90 days to ensure accurate and transparent results.
In this cohort of patients undergoing PD by a single surgeon at the beginning of their career, the overall outcomes are similar and well within the acceptable range for other centers of excellence (8,20-25). There was an increase in the use of neoadjuvant chemotherapy, vein resection, and additional procedures seen in the last year of the study, indicating an increased frequency of complex cancer cases in the practice. Trends in the practice were also seen for the use of LPD and total pancreatectomy. The decreased frequency of LPD over this time period was multifactorial, but mostly related to the increased time needed to perform LPD over OPD. The increased frequency of total pancreatectomy had no clear explanation as this is mainly related to the underlying disease process, which did not significantly vary over the time period either.
Operative times, similar to other studies (7,8,11,12), were found to significantly improve over time. This was true for both LPD and OPD, with both curves mimicking each other with an improvement up to 20–30 cases, respectively (Figure 1). Examining the whole cohort, there was a gradual improvement in operative time up to 50 cases overall, which is similar to Tseng et al. who found an improvement in operative time for three surgeons at around 60 cases (7). EBL was also quite low in our series, and no trends were noted, indicating that there was no learning curve in this respect as previously reported in other studies (7,12).
Only 5 (4.7%) clinically relevant PF were noted in the whole cohort. This is the main driver of postsurgical complications, and no trends were noted over the time period. Other authors have reported similar findings of an acceptable PF rate early within a surgical career (26) or with a new technique (13). Overall clinically relevant morbidity (Clavien III–V) was also low, noted in 14.5% of the entire cohort. This compares favorably to other reported large series of surgical outcomes of PD (8,20-25). The low morbidity seen in this series resulted in low intensive care use, short hospital stay, and low readmission rate.
Other groups have reported on outcomes for high performing Whipple patients as a benchmark comparison for others, including minimally invasive PD (22). In their report, this center noted that 61 of 634 patients (9.6%) met the high performing criteria, and they concluded that minimally invasive PD should be held to this standard to this small subset of patients. In this current study, 49 of 124 patients (40%) met criteria of a high performing Whipple patient and were represented by patients undergoing OPD and LPD equally. While it may be difficult to attribute the overall outcome of a patient undergoing a complex operation simply to the approach used to perform the operation, this study does point out the value of providing benchmarks of perioperative events to use for comparison to help define acceptable outcomes. In a previous single surgeon study, 232 PD were performed with excellent outcomes, and the authors concluded that this provides useful outcome measures from which others can perform self-assessment to lower complication rates (27). We agree with these authors who also point out that there are many factors other than surgical experience necessary to obtain optimal outcomes from this complex operation. Surgeon-related factors often focus on technical expertise, but many other factors, such as the decision on whether to operate or not, judgement regarding the timing, intraoperative decision making regarding the extent of resection, choice of reconstruction method, and management of anatomic or disease severity variability, are key components of this expertise. System-related factors are key components of a high-volume institution and include expert diagnostic radiological services, experienced anesthesia teams, availability of proficient interventional radiological or therapeutic endoscopy, and critical postoperative nursing and recovery pathways.
A multifaceted approach to hepatobiliary surgical training is necessary and critical to avoid causing patients harm due to surgeon inexperience. While it may be difficult to provide a detailed summary, there are several aspects in this particular study that are key ingredients for reproducibility among other centers and surgeons. First, a standardized technique was used to perform PD in this series. This technique had been developed in a prior time period with significant influence from performing LPD, and the OPD procedure had been fashioned after this to the point that both procedures were, for all intents and purposes, exactly the same operation using different instruments. There were no major modifications to this standardized technique to either OPD or LPD by the author over this study time period. The performance of this operation was done repetitively in the fellowship and studied intently with video review. While the actual number of PD cases performed in training may have been less than 50, the time spent in reviewing/editing video recordings and mental preparation for the operation likely contributed an effective surgical training experience of greater than 100 PD. In fact, the ability to video record expert technique may be one of the most beneficial aspects of LPD to the HPB surgical community. This contribution has allowed for greater standardization of the PD procedure across the world than ever before, providing a very positive impact on current surgical training. Reviewing expert minimally invasive PD, whether live or on video, can give a much better understanding of the anatomy, planes of dissection and relation of the pancreas to the neighboring anatomic structures. This fact by itself appears to shorten a learning curve, improve surgical technique, and potentially improve the outcomes for surgeons performing OPD. Subtle but important nuances of the operation, such as uncinate process dissection or pancreaticojejunostomy reconstruction, can be clearly seen and studied in depth on a monitor screen in slow motion when reviewing a video of the operation. This is done in a relaxed environment as opposed to a trainee attempting to observe or perform this operation with limited view and inability to review.
Second, a high-volume center is clearly the optimal place in which to obtain and sustain optimal results. It has been made quite clear in literature that high-volume institutes provide the best chance for a good patient outcome (4). Similarly, the system-related factors that have been mentioned above are crucial for a new surgeon, helping to counteract any propensity for problematic postoperative events in the initial learning phase. An inexperienced surgeon placed in an environment lacking these factors will most assuredly result in a learning curve for both the surgeon and the institution that will result in poor patient outcomes until they are overcome. The availability of an experienced surgeon with the willingness to consult and participate in the surgery as needed is fundamental for minimization of the learning curve during the first years of practice.
Likewise, a third key ingredient is appropriate institutional support and oversight. Resources provided to a surgeon should include experienced operative teams, surgical assistants, and organized support staff. In keeping with the institution’s commitment to excellent surgical care, outcomes were tracked in a thorough fashion, and quality results were monitored and assured.
Lastly, a key component of success for a pancreatic surgeon at the beginning of their career is to perform this operation with relative frequency. This requires entering a practice with colleagues or mentors willing to share a referral base to provide this steady volume of PD. This was highlighted by Fisher et al. who pointed out that no surgeon can begin his/her practice as an experienced high-volume pancreas surgeon (12).
This study’s limitations are that this represents only a single surgeon’s learning experience and initial results under a very particular learning situation. While it would be useful to perform a multi-institutional and multi-surgeon analysis, substantial conclusions regarding the effect of each component of a successful integration of a young pancreatic surgeon into practice would be difficult. The results of this study demonstrate that a learning curve that affects patient outcomes for a pancreatic surgeon may be mitigated by surgeon or institutional measures that optimize the delivery of surgical care. The overall results of the first 124 PD performed by this surgeon compare to those reported by another high-volume center with experienced surgeons who had undergone a period of optimization. In a study reported by Boone et al, technical proficiency and efficiency for performing robotic PD was achieved after performing 80 cases with subsequent improved results for the next 120 cases with regard to operative time, conversion to open surgery, blood loss, LOS, and readmissions (21).
Conclusions
While this study represents a single surgeon experience with inherent limitations, the lessons learned and reported here can have broader application to pancreatic surgeons as they enter the workforce. While it appears that there may be an inevitable learning curve regarding operative time, this study demonstrates that a learning curve that results in a period of increased blood loss, PF, morbidity, or mortality can be avoided by appropriate training and practice environment.
Acknowledgements
The authors would like to acknowledge the assistance provided by Mauricia Buchanan in data collection and Zhou Li with the statistical analysis with support from the Mayo Clinic in Florida Focused Research Team Program.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Internal Review Board (IRB approval # 08-000743).
References
- Whipple AO, Parsons WB, Mullins CR. Treatment of Carcinoma of the Ampulla of Vater. Ann Surg 1935;102:763-79. [Crossref] [PubMed]
- Whipple AO. A reminiscence: pancreaticduodenectomy. Rev Surg 1963;20:221-5. [PubMed]
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011;364:2128-37. [Crossref] [PubMed]
- Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 1999;125:250-6. [Crossref] [PubMed]
- Kotwall CA, Maxwell JG, Brinker CC, et al. National estimates of mortality rates for radical pancreaticoduodenectomy in 25,000 patients. Ann Surg Oncol 2002;9:847-54. [Crossref] [PubMed]
- Rosemurgy A, Cowgill S, Coe B, et al. Frequency with which surgeons undertake pancreaticoduodenectomy continues to determine length of stay, hospital charges, and in-hospital mortality. J Gastrointest Surg 2008;12:442-9. [Crossref] [PubMed]
- Tseng JF, Pisters PW, Lee JE, et al. The learning curve in pancreatic surgery. Surgery 2007;141:694-701. [Crossref] [PubMed]
- Schmidt CM, Turrini O, Parikh P, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg 2010;145:634-40. [Crossref] [PubMed]
- Pecorelli N, Balzano G, Capretti G, et al. Effect of surgeon volume on outcome following pancreaticoduodenectomy in a high-volume hospital. J Gastrointest Surg 2012;16:518-23. [Crossref] [PubMed]
- Enomoto LM, Gusani NJ, Dillon PW, et al. Impact of surgeon and hospital volume on mortality, length of stay, and cost of pancreaticoduodenectomy. J Gastrointest Surg 2014;18:690-700. [Crossref] [PubMed]
- Hardacre JM. Is there a learning curve for pancreaticoduodenectomy after fellowship training? HPB Surg 2010;2010:230287. [PubMed]
- Fisher WE, Hodges SE, Wu MF, et al. Assessment of the learning curve for pancreaticoduodenectomy. Am J Surg 2012;203:684-90. [Crossref] [PubMed]
- Kim SC, Song KB, Jung YS, et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc 2013;27:95-103. [Crossref] [PubMed]
- Jeyarajah DR, Patel S, Osman H. The Current State of Hepatopancreatobiliary Fellowship Experience in North America. J Surg Educ 2015;72:144-7. [Crossref] [PubMed]
- Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 2012;215:810-9. [Crossref] [PubMed]
- DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 2006;244:931-7; discussion 937-9. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 2006;10:1199-210;discussion 1210-1. [Crossref] [PubMed]
- Boone BA, Zenati M, Hogg ME, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 2015;150:416-22. [Crossref] [PubMed]
- Lee GC, Fong ZV, Ferrone CR, et al. High performing whipple patients: factors associated with short length of stay after open pancreaticoduodenectomy. J Gastrointest Surg 2014;18:1760-9. [Crossref] [PubMed]
- Tee MC, Croome KP, Shubert CR, et al. Laparoscopic pancreatoduodenectomy does not completely mitigate increased perioperative risks in elderly patients. HPB (Oxford) 2015;17:909-18. [Crossref] [PubMed]
- Bentrem DJ, Yeh JJ, Brennan MF, et al. Predictors of intensive care unit admission and related outcome for patients after pancreaticoduodenectomy. J Gastrointest Surg 2005;9:1307-12. [Crossref] [PubMed]
- Vollmer CM Jr, Lewis RS, Hall BL, et al. Establishing a quantitative benchmark for morbidity in pancreatoduodenectomy using ACS-NSQIP, the Accordion Severity Grading System, and the Postoperative Morbidity Index. Ann Surg 2015;261:527-36. [Crossref] [PubMed]
- Noda H, Kamiyama H, Kato T, et al. Risk factor for pancreatic fistula after pancreaticoduodenectomy performed by a surgeon during a learning curve: analysis of a single surgeon's experiences of 100 consecutive patients. Hepatogastroenterology 2012;59:1990-3. [PubMed]
- Traverso LW, Shinchi H, Low DE. Useful benchmarks to evaluate outcomes after esophagectomy and pancreaticoduodenectomy. Am J Surg 2004;187:604-8. [Crossref] [PubMed]
Cite this article as: Tsamalaidze L, Stauffer JA. Pancreaticoduodenectomy: minimizing the learning curve. J Vis Surg 2018;4:64.