Adult pulmonary intralobar sequestrations: changes in the surgical management
Introduction
Pulmonary sequestrations are congenital malformations in which a portion of the pulmonary parenchyma is vascularized by a systemic artery, arising most often from the thoracic or subdiaphragmatic descending aorta. They result from a defect in the obliteration of the splanchnic arteries during embryogenesis (1). They are divided into extralobar (ELS) and intralobar (ILS) sequestrations.
- ELS are usually diagnosed in the newborn, or even in the peri-natal period. They are characterized by a pulmonary territory independent of the normal parenchyma, vascularized by an artery from the aorta, draining into a systemic vein and without connection to the tracheobronchial tree. They are almost always associated with other malformations, especially cardiac ones;
- ILS are more common in adults. They are characterized by a pulmonary territory that communicates with the respiratory tree but is vascularized by a systemic artery coming from the descending thoracic abdominal aorta or even the celiac trunk, with normal venous drainage into the pulmonary veins. They are usually located in the basal segments of the lower lobes. They are covered with a normal visceral pleura shared with the adjacent healthy pulmonary parenchyma. ILS are often detected in adulthood after pulmonary infections and/or haemoptysis, and more rarely, after cardiac insufficiency by venous return overload (2). They are sometimes discovered accidentally or confused with another condition. Until recently, the treatment of ILS was exclusively surgical and was based on the interruption of the systemic artery associated with lobectomy by open chest surgery. Other surgical or non-surgical treatments have recently been proposed, aiming to be less invasive and to spare the pulmonary parenchyma. The aim of this work is to determine if the surgical management of these lesions has evolved over the last years.
Methods
Between January 2000 and December 2016, we retrospectively reviewed the records of patients who underwent surgery for pulmonary sequestration in our department. Clinical and demographic data including patients age, sex, preoperative symptoms, type of surgery, type of resection and postoperative course were analyzed (Table 1). The approach was a posterolateral thoracotomy (PLT group) or an exclusive thoracoscopy (TS group) according to a standardized technique previously described in details (3). Briefly, the procedure was performed under general anesthesia with split ventilation using a double-lumen endotracheal tube. Patients were positioned in lateral decubitus as for a thoracotomy. The surgeon stood anterior to the patient for left side resection and posteriorly for right sided ones. Two monitors were used and the deflectable thoracoscope was placed on a mechanical scope holder. Only specifically designed endoscopic instruments for video-assisted thoracoscopic surgery (VATS) major resections were used. Trocars had a diameter ranging between 3 and 15 mm. Larger vessels were divided with endostaplers, while haemostasis of small caliber vessels was performed with clips, with a bipolar vessel sealing device or with a combination of both methods. The intersegmental plane was divided by a combination of bipolar sealing device (for its peripheral and thin portion) and stapling (for its central and thick portion) using 4.8 mm staples for segmentectomies. No utility incision was used. On completion of the pulmonary resection, the specimen was wrapped into an endobag and retrieved through one of the port sites that was enlarged, depending on the specimen size. The use of a rib spreader was never required for specimen extraction. In most cases, only one chest tube was placed through one of the port site.

Full table
Preoperatively, the aberrant artery was identified using contrast-enhanced tomography (CT). In both groups, the control of the aberrant artery was accomplished using clips or endostaplers (Figure 1) with temporary aortic clamping if necessary (Figure 2). The chest tube removal was decided when no air leakage was present and the daily output inferior to 400 cc.

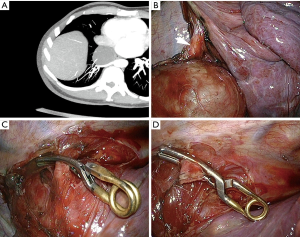
Results
Between 2000 and 2016, 18 patients with pulmonary sequestration were operated on in our department. Sex repartition was equivalent: 9 females and 9 males. Mean age was 36.3 years (range, 14.0–54.0 years). Fifteen patients (83.3%) had respiratory symptoms, whereas only three patients (16.7%) were asymptomatic (Table 1).
All ILS were in the lower lobes, more frequently on the left side (n=12, 66.7%) than on the right one (n=6). In the thoracotomy group (11 patients, one of whom was converted due to dense adhesions), all patients underwent a lobectomy (Figure 3), while in the thoracoscopy group (seven patients), five patients underwent a sublobar resection. Among them, either an atypical resection or an anatomical segmentectomy (Figure 4) were performed. In the thoracoscopy group, the systemic artery was always identified on an injected CT scan before the operation except in one patient, in whom the diagnosis of sequestration was not evoked, with a misinterpretation of the CT scan.
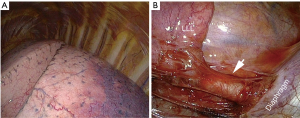
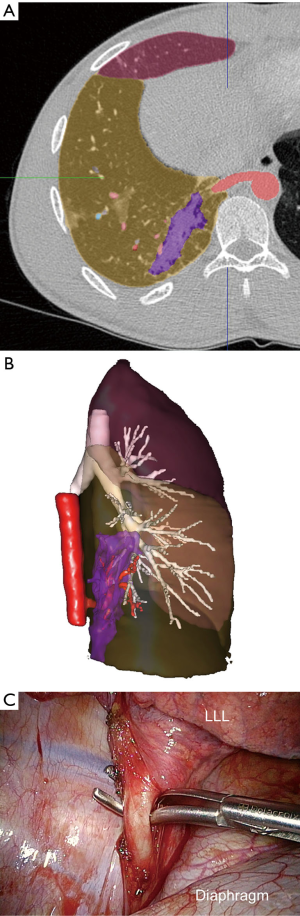
Pathological examination confirmed the ILS diagnosis in all cases. In one patient, two abnormalities were found in the same lobe: a bronchogenic cyst and sequestration.
No mortality was observed. In the PLT group, 5 patients (45%) experienced minor complications: pleural effusion (n=2), pneumonia (n=1), prolonged air leakage (n=1) and chylothorax (n=1). In the thoracoscopy group, one patient (14%) presented a chylothorax requiring further surgery performed by thoracoscopy.
The mean hospital stay was 7.4 days (range, 5–11 days) in the thoracotomy group versus 5.4 days (range, 3–8 days) in the thoracoscopy group.
Discussion
ILS complications can be severe and are usually of two types: infectious or hemorrhagic (5,6). Therefore, even when the symptoms are moderate or when they are accidentally diagnosed, a curative treatment is proposed. In the literature, it is generally accepted that resection is the treatment of choice for ILS and the standard treatment remains lobectomy by thoracotomy. Thus, in a large cohort published in 2016, out of 58 patients undergoing surgery, 56 had a lobectomy, by thoracotomy in 72% of cases (7). In the majority of cases, ILS are located in the basal segments of the inferior lobe with normal adjacent parenchyma. A parenchyma-sparing surgery should therefore be considered. Pediatric surgeons who are aware of the importance of sublobar pulmonary resection, not only to spare respiratory function but also to allow for the most normal development of the thoracic cage which avoids scoliosis, have proposed performing segmentectomies (8,9), or even wedge resections (9,10). Bagrodia et al. (8) did not record more recurrence after segmentectomy than after lobectomy in a large comparative series of surgical treatment of pulmonary malformations, including a majority of sequestrations. Fascetti-Leon et al. have performed 54 sublobar resections (segmentectomies or wedge resection) for congenital pulmonary malformations and conclude that it is appropriate when the lesions are peripheral and limited in size (9). Lin et al. reported a series of 26 patients operated for ILS. Six had a wedge resection whereas 19 underwent a lobectomy. They conclude that in selected cases, wedge resection is a valid alternative (10). It is surprising that no patient in this series had a segmentectomy which is a better alternative than wedge resection which is rarely possible under satisfactory conditions due to the large lesion size. However, wedge resection can be proposed in the case of a small and very peripheral lesion (9,10).
Embolization of the ILS systemic artery has been proposed, mainly in pediatry (11). However, in a study involving 73 children and comparing surgical resection (n=31) versus embolization (n=42), the complications and failure rate was significantly higher in the embolization group, in which only three complete lesions regression were observed. Four children experienced post-embolization sepsis and three had to undergo secondary surgery (12). The authors concluded that surgical resection was the most effective treatment. In adults, embolization of the systemic artery has also been reported, but as clinical cases or in very short series, without any valuable conclusions (13). Sometimes, the bulky size of these arteries (Figure 5) and/or their multiple numbers (2) raise doubts about the long-term effectiveness of embolization. In case of massive hemoptysis, embolization followed by surgical resection appears to be effective, but these are only isolated cases (1,14).
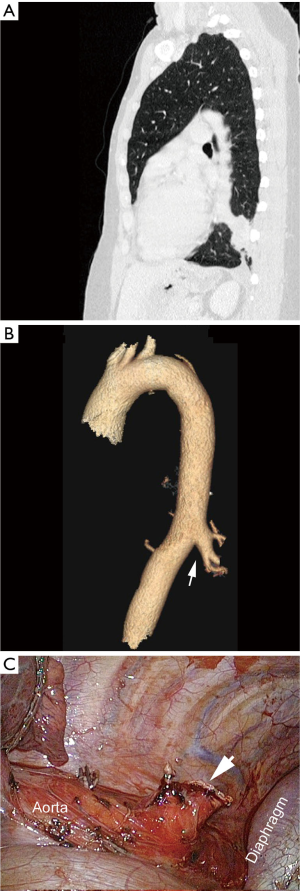
In conclusion:
- Without large series with prolonged follow-up, there is currently no argument for proposing embolization as the only curative treatment of an adult ILS, especially in the case of a large systemic artery and/or extensive parenchymal lesions;
- If surgical resection is retained, there is no evidence to suggest that a lobectomy is preferable to sublobar resection, unless the sequestration involves almost all of a lobe;
- Since perioperative morbidity of sublobar resection is reduced when performed by thoracoscopy rather than open chest surgery (15,16), we conclude, as other authors (8,9) that ILS can be safely treated by anatomical segmentectomies that should be performed, whenever possible, without opening the chest.
Acknowledgements
None.
Footnote
Conflicts of Interest: D Gossot is consultant for an instrument manufacturer (Delacroix Chevalier). The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Ethics Committee of CEPAR (No. 1682873 v 0). Written informed consent was obtained from all patients.
References
- Avsenik J, Štupnik T, Popovič P. Endovascular embolization prior to surgical resection of symptomatic intralobar pulmonary sequestration in an adult. Eur J Radiol Open 2015;3:12-5. [Crossref] [PubMed]
- Wang S, Ruan Z, Liu F, et al. Pulmonary sequestration: angioarchitecture evaluated by three-dimensional computed tomography angiography. Thorac Cardiovasc Surg 2010;58:354-6. [Crossref] [PubMed]
- Gossot D. Atlas of endoscopic major pulmonary resections. Paris: Springer-Verlag, 2018:170.
- Traibi A, Seguin-Givelet A, Gossot D, et al. Example of stapling a systemic artery. Asvide 2018;5:303. Available online: http://www.asvide.com/article/view/23689
- Hofman FN, Pasker HG, Speekenbrink RG. Hemoptysis and massive hemothorax as presentation of intralobar sequestration. Ann Thorac Surg 2005;80:2343-4. [Crossref] [PubMed]
- Ferland N, Couture C, Provencher S. Near-fatal haemoptysis as presentation of a giant intralobar pulmonary sequestration. Eur Respir Rev 2015;24:155-6. [Crossref] [PubMed]
- Wang LM, Cao JL, Hu J. Video-assisted thoracic surgery for pulmonary sequestration: a safe alternative procedure. J Thorac Dis 2016;8:31-6. [PubMed]
- Bagrodia N, Cassel S, Liao J, et al. Segmental resection for the treatment of congenital pulmonary malformations. J Pediatr Surg 2014;49:905-9. [Crossref] [PubMed]
- Fascetti-Leon F, Gobbi D, Pavia SV, et al. Sparing-lung surgery for the treatment of congenital lung malformations. J Pediatr Surg 2013;48:1476-80. [Crossref] [PubMed]
- Lin ZW, Gu J, Xu ST, et al. Video-Assisted Thoracoscopic Surgery for Intralobar Pulmonary Sequestration: Wedge Resection Is Feasible in Limited Peripheral Lesions. Thorac Cardiovasc Surg 2016;64:456-60. [Crossref] [PubMed]
- Brown SC, De Laat M, Proesmans M, et al. Treatment strategies for pulmonary sequestration in childhood: resection, embolization, observation? Acta Cardiol 2012;67:629-34. [Crossref] [PubMed]
- Cho MJ, Kim DY, Kim SC, et al. Embolization versus surgical resection of pulmonary sequestration: clinical experiences with a thoracoscopic approach. J Pediatr Surg 2012;47:2228-33. [Crossref] [PubMed]
- Leoncini G, Rossi UG, Ferro C, et al. Endovascular treatment of pulmonary sequestration in adults using Amplatzer® vascular plugs. Interact Cardiovasc Thorac Surg 2011;12:98-100. [Crossref] [PubMed]
- Goto T, Toya K, Wakaki M, et al. Resection of intralobar pulmonary sequestration after coil embolization of aberrant arteries: report of a case. Surg Today 2013;43:923-5. [Crossref] [PubMed]
- Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014;148:637-43. [Crossref] [PubMed]
- Traibi A, Grigoroiu M, Boulitrop C, et al. Predictive factors for complications of anatomical pulmonary segmentectomies. Interact Cardiovasc Thorac Surg 2013;17:838-44. [Crossref] [PubMed]
Cite this article as: Traibi A, Seguin-Givelet A, Brian E, Grigoroiu M, Gossot D. Adult pulmonary intralobar sequestrations: changes in the surgical management. J Vis Surg 2018;4:62.


