Aortic valve insufficiency in aortic root aneurysms: consider every valve for repair
Introduction
Aortic insufficiency (AI) is a common condition with a prevalence of 13% in men and 8% in women in the Framingham study (1). Patients presenting with AI and aortic root aneurysm have traditionally been treated with a composite root and valve replacement (Bentall procedure) with either biologic or mechanical valve prosthesis (2). The Bentall procedure has been the gold standard for decades. Whereas early outcomes have been excellent, patients incur the cumulative long-term risks associated with prosthetic valve complications. These risks include thromboembolism, prosthetic valve endocarditis, structural and non-structural valve deterioration and dysfunction requiring reoperation, and the inconvenience and risks of anticoagulation in the case of mechanical valves. In the case of the mitral valve, these concerns became the impetus for the development of mitral valve repair in the 1980’s (3). Repairing the valve while preserving the patient’s own tissues obviated the need for anticoagulation and reduced prosthesis-related complications (4). Similarly, preserving and repairing the aortic valve (AV) promises to substantially reduce these risks. The first important milestone in the development of AV repair was the preservation of the AV in the context of aortic root pathology and can be traced back to the early 1990’s with the description of valve sparing root replacement (VSRR) using the reimplantation and remodeling techniques by David and Yacoub (5-7). In the last 15 years, the development of cusp repair techniques (8,9), emergence of a repair-oriented classification system for AI (10), and reporting of long-term outcomes (11-14) contributed to propel the field of AV repair forward. With these developments in mind we are at a point where every patient who presents with AI and an aortic root aneurysm should at least be considered and evaluated for a valve sparing or valve repair procedure. Herein, we review these developments, which lend credence to an aggressive valve preservation and repair strategy.
AV anatomy and function
An in-depth understanding of AV anatomy and function is paramount to its successful preservation and repair. A complex interaction between the AV cusps and the annulus is the basis of a normally functioning AV. Although surgeons commonly refer to the annulus as a single entity, in reality, the functional aortic annulus (FAA) is made of three components: the sinotubular junction (STJ), the ventriculo-aortic junction (VAJ), and the anatomic crown-shaped annulus on which the cusps insert. In a normal AV, the cusps coapt at the center of the AV orifice and midway between the STJ and VAJ. An essential tenet of AV repair is that lesions of the FAA and the cusps should both be addressed at the time of valve repair.
Classification of AI
Without an understanding of the mechanisms of AI, choosing an appropriate surgical repair technique becomes challenging. The emergence of a repair-oriented classification of AI (Figure 1) has allowed AV repair to transition from an art performed by a few skilled surgeons to a systematic and scientific process that can potentially be adopted by most surgeons. First, it provides a framework to understand the mechanisms of AI in a specific patient and choose an appropriately tailored technique or combination of techniques to restore normal valve function. Second, it provides us with a universal vocabulary, which helps with communication in both the clinical and research settings, much like the Carpentier classification did for mitral valve repair (3). Aortic regurgitation associated with normal cusp motion is designated as type I. This is mostly due to lesions of the FAA with type Ia due to dilatation of the ascending aorta and STJ, type Ib due to dilatation of the VAJ and the STJ, type Ic due to dilatation of the VAJ, and type Id due to cusp perforation without any FAA lesions as in the example of infective endocarditis. Type II AI is due to cusp prolapse secondary to excessive cusp tissue or commissural disruption. Type III AI is due to leaflet restriction from calcification, thickening, or fibrosis of the cusps as can be found in bicuspid, degenerative, or rheumatic valve disease. The focus of this review is AI secondary to type Ib with concomitant type II and type III lesions (non-shaded columns of Figure 1).
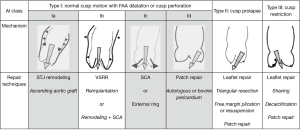
Indications for AV preservation and repair
Indications for AV repair for AI in the context of an aortic root aneurysm fall into two broad categories. First, for the AI, the indications for repair are the same as for AV replacement (15) including severe symptomatic AI, asymptomatic severe AI with left ventricular (LV) systolic dysfunction (ejection fraction <50%), and severe asymptomatic AI with normal LV systolic function but with severe LV dilatation (LV end systolic diameter >50 mm). Second, for patients with primary aortic pathology, indication for valve preservation and repair is largely driven by aortic size, growth rate, or associated bicuspid aortic valve (BAV), genetic and familial conditions (16,17). Technically, all patients with primary AI are potential candidates for repair and should be assessed for it pre- and intra-operatively. However, the success of that repair is largely determined by the quality of the cusp tissue available and the surgical expertise and experience in AV repair. Several other factors contribute to the success of preservation and repair the AV, which will be discussed in the next sections of the manuscript.
AV repair: selection, techniques, and outcomes
Patient selection
Transesophageal echocardiography provides the first opportunity for thorough valve assessment and provides important information on mechanisms of AI and valve reparability. Key aspects to consider include jet origin and direction, end-diastolic measurements of the VAJ, STJ, and Sinuses of Valsalva, cusp thickness, mobility, and presence of calcification. However, visual inspection intra-operatively remains the final arbiter as to whether a valve will be amenable to repair. Cusp tissue quality is perhaps the most important factor in deciding whether preservation and repair of the AV is feasible. Heavily calcified or fibrotic cusps or ones destroyed by infection usually preclude repair. Similarly, severely dilated STJs tend to stretch the cusp free margin leading to stress fenestrations along the commissures, which makes a durable AV repair unlikely. Decreased cusp geometric height (<16 mm) is another factor that may preclude a good AV repair, as the annulus would have to be reduced excessively to allow for adequate cusp coaptation. Sievers type 1 BAVs (18) with commissural angles <140° also can be challenging to repair and may require techniques such as tricuspidization. In the context of a root aneurysm and one of the above cusp findings, a Bentall procedure may be the preferred technique (19).
Techniques of AV repair
Broadly speaking, surgical techniques for AV repair can be divided into two categories: techniques for the aortic root and annular remodeling and cusp repair techniques. Patients presenting with AI associated with a root aneurysm (type Ib), addressing the aortic root by performing a VSRR is mandatory. Either the reimplantation or remodeling plus annuloplasty techniques can be used. We prefer the reimplantation technique (20) as it provides better stabilization of the VAJ (Figure 2). Remodeling of the aortic root is postulated to better preserve annular dynamics during the cardiac cycle, though the impact on clinical outcome remains uncertain (22). However, long-term results of the remodeling technique have not been as good as the reimplantation technique, especially in patients with aortic root aneurysms associated with BAV insufficiency and genetic syndromes (12,23-25).

Cusp prolapse is the most frequent cusp pathology encountered and is caused by excess free margin length, especially when VSRR using the reimplantation technique is used to reduce the annular dimensions. Correction of slight prolapse can be achieved with a central free margin plication (Figure 3) using a small caliber Prolene suture placed in the center of the free margin to plicate, shorten, and reduce its length and therefore raise its height (9). An alternative technique is free margin resuspension performed by passing a PTFE suture over and over the free margin and exteriorizing the suture at the commissures (Figure 3). Pulling on this suture has the effect of performing multiple plications along the free margin thereby shortening and raising it (9). For larger degrees of prolapse correction, a small portion of the cusp requires resection with primary reapproximation.

BAVs can be insufficient due to prolapse (type II AI), typically in type 0 BAV or type 1 BAV with a short fibrous raphe and well-developed conjoint cusp. In these scenarios, the above-mentioned cusp repair techniques still apply, with the addition of shaving a fibrous raphe off while preserving the cusp if amenable. BAVs can also be insufficient due to conjoint cusp restriction (type III AI) usually in type I BAV with a rigid and calcified raphe. In this scenario, either resection of the raphe with primary reapproximation, or resection and cusp restoration with patch material is usually required. Patch material has also been used for the tricuspidization of BAVs. However, the use of patch material in AV repair is a predictor of long-term repair failure (27). Figure 3 demonstrates shaving of a piece of calcium that is causing cusp restriction.
Outcomes of AV preservation and repair
The absence of an ideal prosthetic AV is perhaps the main driving force behind AV repair. Structural and non-structural valve degeneration is the Achilles heel of bioprosthetic valves in the typically younger AV repair population with a median time to explant of less than 8–10 years (28). Mechanical valves carry a risk of thromboembolism and require the inconvenience and burden of anticoagulation with a 1–2% annual risk of serious hemorrhage (28). They do not guarantee lifelong durability with a cumulative risk of valve-related events of 5%/patient year which can accrue over the lifespan of a young patient (28). Lastly prosthetic valve endocarditis is a devastating disease with high morbidity and mortality.
In contrast, AV repair has been shown in multiple studies to have lower risk of valve-related events, while maintaining low rates of mortality. In one cohort of 475 patients, AV repairs were associated with low risk of thromboembolism, bleeding, and endocarditis with linearized rates of 1.1%, 0.23%, and 0.19% per year, respectively. Thirty-day mortality rate was 0.8% (13). Similarly, a study of 640 AV repair patients demonstrated a low incidence of mortality (0.8%), thromboembolism (0.2%/year) and endocarditis (0.16%/year) with a 10-year freedom from all valve-related complications of 88% (11). The results were similar for 122 consecutive BAV repairs with an 8-year freedom from bleeding, thromboembolism and endocarditis of 96% (27). There were no operative mortalities in this report.
The durability of repaired valves remains both an important limitation and also an opportunity for improvement in patients undergoing AV repair. David et al.’s group reported their quarter of a century experience of AV sparing operations in 371 patients. Survival at 18 years was 77%, keeping in mind that at least half the patients had other cardiovascular disease such as type A dissection or coronary artery disease. Freedom from reoperation on the AV and greater than mild AI at 18 years was 95% and 78%, respectively (12). The Cleveland Clinic reported their long-term outcomes of BAV repair in 728 patients (14). Their hospital mortality was 0.41%. The 10-year survival and freedom from reoperation were 94% and 78%, respectively. After an initial postoperative peak, the risk of reoperation fell rapidly thereafter to 2.6%/year up to 15 years. The most common reason for reoperation was cusp prolapse (38%), followed by aortic stenosis or insufficiency (17%) and root aneurysms (15%). A contemporary systematic review of AV preservation and repair identified 17 studies involving 2,891 patients (29). Pooled early mortality was 2.6%. Median linearized rates of thromboembolism and endocarditis were 0.52%/patient-year and 0.23%/patient-year, respectively. Late AV re-intervention occurred at a rate of 2.4%/patient-year. The median 5-year re-intervention and late recurrent AI >2+ were 92% and 88%, respectively. These are acceptable numbers when compared to the median durability of 8–10 years of bioprosthetic valves (i.e., 50% reoperation rate at 8–10 years) implanted in younger patients or the risks of thromboembolism, anticoagulation-related hemorrhage, and endocarditis associated with mechanical valves.
As the field of AV repair matures, its scope is extending from VSRR in AVs with normal cusps to ones with significant cusp pathology. During this evolution, important predictors of repair failure are identified, yielding areas in need of further refinement. For example, type III AI due to cusp restriction is a risk factor for recurrent AI and reduced repair durability. We have previously shown that patients with cusp restriction have a 5-year reoperation risk of 15%, compared to 5% in patients presenting with type I and II AI (10). We have also shown that leaving patients with a dilated annulus post repair is a risk factor for repair failure, especially in patients with BAVs. These patients typically require a 4–5 mm of annular diameter reduction for a successful repair. We found that VSRR using the reimplantation technique provides the most robust annuloplasty in BAVs (24,30). Others have shown that although freedom from AI >2+ in the mid- and long-term is similar in repaired BAVs using root reimplantation versus subcommissural annuloplasty, freedom from AI >1+ was significantly higher in the root reimplantation group (92% vs. 62%). Once again, this is presumably due to more robust root stabilization in the root reimplantation group. Another risk factor for repair failure is commissural configuration in BAVs. Commissural angles <140° in particular are associated with repair failure (31). Lastly, the use of patch material for cusp reconstruction or augmentation is associated with late repair failures as well.
Future of AV repair
The evolution of AV repair into an established discipline mirrors that of mitral valve repair with a 10–20-year delay. The delay is multifactorial. First, in contrast to mitral valve, the most common AV pathology is degenerative calcific aortic stenosis, which for the most part precludes repair and mandates AV replacement. Second, unlike the mitral valve, the surgeon’s view of the AV is from its outflow side, making it very difficult for surgeons to visually assess and test the valve in its pressurized state. Preoperative echocardiographic assessment is perhaps even more important when venturing into AV repair. Third, as mentioned previously, the FAA is not a single entity like the mitral valve, but rather made up of several components that interact in a complex manner for the normal functioning of the AV. Therefore, annuloplasty of the AV requires evaluating and addressing all the components of the FAA simultaneously.
Several important challenges lie ahead for AV repair to become established as a discipline. First, the repertoire of surgical materials and techniques must expand to allow the surgeon to refine and tailor repair strategies to individual patients with various permutations of cusp and FAA lesions. Techniques and materials to address isolated VAJ dilatation in the context of a non-dilated root is one area requiring further refinement. Currently valve sparing root replacement using the reimplantation technique provides the most stability to the VAJ but may be too aggressive in isolated VAJ dilatation commonly seen in BAVs. External annuloplasty of the VAJ is increasingly being used for this indication. Development of better materials for cusp augmentation and reconstruction is another area requiring improvement. The most commonly used materials today, bovine or autologous pericardium, are associated with limited long-term durability. The ideal material would have excellent handling properties, be available off the shelf, endure hemodynamic stresses without degeneration or calcification, and would not trigger an immune response. A third area requiring innovation would be designing a device to visually assess and test a pressurized AV pre- and post-repair in a reproducible and accurate manner.
Second, there is a need for well-conducted long-term outcome studies in AV repair. The existing literature is limited to several single-center, single surgeon studies with heterogeneity in surgical technique, inadequate description of the population studied, and with loss to follow-up and incomplete or inadequate reporting of valve-related events. Furthermore, more studies comparing AV repair and VSRR with AV replacement and Bentall procedure are required. As more surgeons and centers adopt AV repair and with increasing volumes, studies comparing various repair techniques in specific patient populations will be the ultimate refinement required.
Third, and perhaps the biggest challenge, will be the dissemination of surgical technique around the world. Critics of AV preservation and repair will argue that these techniques are too complex for most surgeons to adopt and most data is emerging from only a few surgeons and centers worldwide. However, we have previously shown that AV repair is reproducible and that procedural safety and efficiency improves with experience with a learning curve of approximately 40–60 cases (32). Animal, cadaveric, and computer simulation models may be important adjuncts to facilitate education.
Lastly, advances in imaging and computer technology now permit the construction of finite element models of the AV from real patients data obtained from pre-operative imaging. Various surgical techniques can then be implemented within these models to assess and predict the outcome of certain repair strategies. Advancement in this ‘virtual surgery’ platform can facilitate patient-specific surgical planning and tailored repair strategies, which can make AV repair more predictable.
Conclusions
As more data emerges showing the safety, low incidence of valve-related complications, and favorable long-term data, valve preservation and repair is emerging as an attractive alternative in younger patients presenting with AI and an aortic root aneurysm. Despite these results, there is a need for further refinement of techniques, larger multicenter studies with longer follow-up, and dissemination of knowledge and expertise in order to improve valve repair durability in various valve pathologies and AI mechanisms. However, with the current knowledge, every patient presenting with AI and aortic root aneurysm should be considered and evaluated for a valve preserving procedure in centers with sufficient AV repair experience.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999;83:897-902. [Crossref] [PubMed]
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Braunberger E, Deloche A, Berrebi A, et al. Very long-term results (more than 20 years) of valve repair with carpentier's techniques in nonrheumatic mitral valve insufficiency. Circulation 2001;104:I8-11. [Crossref] [PubMed]
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21. [PubMed]
- David TE, Feindel CM, Bos J. Repair of the aortic valve in patients with aortic insufficiency and aortic root aneurysm. J Thorac Cardiovasc Surg 1995;109:345-51. [Crossref] [PubMed]
- Sarsam MA, Yacoub M. Remodeling of the aortic valve anulus. J Thorac Cardiovasc Surg 1993;105:435-8. [PubMed]
- Aicher D, Langer F, Adam O, et al. Cusp repair in aortic valve reconstruction: does the technique affect stability? J Thorac Cardiovasc Surg 2007;134:1533-8. [Crossref] [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. Cusp prolapse repair in trileaflet aortic valves: free margin plication and free margin resuspension techniques. Ann Thorac Surg 2009;88:455-61. [Crossref] [PubMed]
- Boodhwani M, de Kerchove L, Glineur D, et al. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009;137:286-94. [Crossref] [PubMed]
- Aicher D, Fries R, Rodionycheva S, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2010;37:127-32. [Crossref] [PubMed]
- David TE, Feindel CM, David CM, et al. A quarter of a century of experience with aortic valve-sparing operations. J Thorac Cardiovasc Surg 2014;148:872-9. [Crossref] [PubMed]
- Price J, De Kerchove L, Glineur D, et al. Risk of valve-related events after aortic valve repair. Ann Thorac Surg 2013;95:606-12. [Crossref] [PubMed]
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014;97:1539-47. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-643. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Appoo JJ, Bozinovski J, Chu MW, et al. Canadian Cardiovascular Society/Canadian Society of Cardiac Surgeons/Canadian Society for Vascular Surgery Joint Position Statement on Open and Endovascular Surgery for Thoracic Aortic Disease. Can J Cardiol 2016;32:703-13. [Crossref] [PubMed]
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226-33. [Crossref] [PubMed]
- McCarthy FH, Bavaria JE, McDermott KM, et al. At the Root of the Repair Debate: Outcomes After Elective Aortic Root Replacements for Aortic Insufficiency With Aneurysm. Ann Thorac Surg 2016;102:1199-205. [Crossref] [PubMed]
- Boodhwani M, de Kerchove L, El Khoury G. Aortic root replacement using the reimplantation technique: tips and tricks. Interact Cardiovasc Thorac Surg 2009;8:584-6. [Crossref] [PubMed]
- Al-Atassi T, Boodhwani M. Valve sparing root replacement using the reimplantation technique to repair type IB aortic insufficiency. Asvide 2018;5:300. Available online: http://www.asvide.com/article/view/23548
- Leyh RG, Schmidtke C, Sievers HH, et al. Opening and closing characteristics of the aortic valve after different types of valve-preserving surgery. Circulation 1999;100:2153-60. [Crossref] [PubMed]
- David TE, David CM, Manlhiot C, et al. Outcomes of Aortic Valve-Sparing Operations in Marfan Syndrome. J Am Coll Cardiol 2015;66:1445-53. [Crossref] [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2011;142:1430-8. [Crossref] [PubMed]
- Erasmi AW, Sievers HH, Bechtel JF, et al. Remodeling or reimplantation for valve-sparing aortic root surgery? Ann Thorac Surg 2007;83:S752-6; discussion S785-90.
- Al-Atassi T, Boodhwani M. Cusp repairs including cusp free margin plication and resuspension for type II aortic insufficiency and calcium shaving for type III aortic insufficiency. Asvide 2018;5:301. Available online: http://www.asvide.com/article/view/23550
- Boodhwani M, de Kerchove L, Glineur D, et al. Repair of regurgitant bicuspid aortic valves: a systematic approach. J Thorac Cardiovasc Surg 2010;140:276-284.e1. [Crossref] [PubMed]
- Chan V, Malas T, Lapierre H, et al. Reoperation of left heart valve bioprostheses according to age at implantation. Circulation 2011;124:S75-80. [Crossref] [PubMed]
- Saczkowski R, Malas T, de Kerchove L, et al. Systematic review of aortic valve preservation and repair. Ann Cardiothorac Surg 2013;2:3-9. [PubMed]
- Navarra E, El Khoury G, Glineur D, et al. Effect of annulus dimension and annuloplasty on bicuspid aortic valve repair. Eur J Cardiothorac Surg 2013;44:316-22. [Crossref] [PubMed]
- Aicher D, Kunihara T, Abou Issa O, et al. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011;123:178-85. [Crossref] [PubMed]
- Malas T, Saczkowski R, Sohmer B, et al. Is Aortic Valve Repair Reproducible? Analysis of the Learning Curve for Aortic Valve Repair. Can J Cardiol 2015;31:1497.e15-22. [Crossref] [PubMed]
Cite this article as: Al-Atassi T, Boodhwani M. Aortic valve insufficiency in aortic root aneurysms: consider every valve for repair. J Vis Surg 2018;4:60.


