Enhanced Recovery After Surgery: a patient centered process
Introduction and historical notes
The Enhanced Recovery After Surgery (ERAS) is a model of care introduced in 1997 by a group of general surgeons from Northern Europe led by Henrik Kehlet (1-4) with a background experience on colorectal fast track surgery. They formed a research group with the aim to explore the ultimate care pathway for patients undergoing open colorectal procedures, and specifically, to implement strategies in the effort to decrease the incidence of postoperative ileus affecting cost and length of hospital stay.
The core of this approach was to reduce the body’s reaction to surgical stress by optimizing the perioperative nutritional status, promoting analgesia without opioids and early postoperative feeding (3).
The group grew over time with colleagues joining in from several other countries and surgical specialties and the first papers reported important improvements in time and quality of recovery after various kinds of surgery (4).
In 2010, following the significant and growing successes reported by these studies, the “ERAS® Society” was officially created and registered in Sweden as an international non-profit medical academic society with members from different professions involved in surgical care (4).
The activities of ERAS Society include publishing and updating a range of guidelines by aid of experts from around the world, continuing to develop guidelines addressing additional surgical specialties, running an annual international congress since 2012, and especially developing the “ERAS implementation program” (EIP). In the EIP teams of surgeons, anaesthetists, nurses, and allied health professionals come together in workshops over a period of 8–10 months and are coached while implementing ERAS in their own unit (4).
The current ERAS Society implementation program was initiated in Sweden, then spread out in the Netherlands, United Kingdom, and Switzerland and later extended to Canada, Australasia, United States, France, Spain, and Latin America (4).
The EIP provides the ERAS Interactive Audit System (EIAS), intended for real-time quality control including a hourly updated database that became a powerful tool for clinical research of the health care providers involved with the ERAS Society network (4).
Several reports from different centers described relevant savings implementing ERAS into daily care (4).
ERAS is not a single and rigid protocol but is a method, a “modus operandi”, a new way of multidisciplinary teamwork with readiness to make changes as knowledge evolves, i.e., a revolution of medical-scientific thought (4).
State of the art and future challenges
Recent reviews of the literature about ERAS in colorectal surgery, general surgery, thoracic surgery, urology, and gynaecology reported that the implementation of ERAS protocols has not been uniform across surgical specialties (3,5).
Overall, ERAS protocols produced improvements in patient outcomes, especially in the reduction of hospital stay, while complication and re-admission rates were significantly reduced only for colorectal surgery (3,5).
Simply elaborating and establishing a protocol (Table 1) is not enough and much more efforts and changes are needed to achieve the aim to offer a sustainable improvement in the overall quality of patient care.
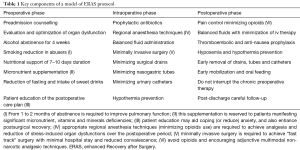
Full table
More specifically (4,5): first thing to do is to create a multidisciplinary team consisting of 4 to 8 people including a surgeon, an anaesthesiologist, a nurse and an administrative employee. Particularly, it would be necessary for the nurse to be able to dedicate at least 50% of the working time to the ERAS protocol. The aforementioned team must adhere to the EIP, carefully following the directives and implementing these protocols in its own units and/or hospitals. The team is trained by expert specialists identified and directly certified by ERAS Society. The training period lasts 8–10 months and is structured in 4 workshops. Between the various workshops, the staff defines the “action periods” during which the professional-students have specific tasks to do or goals to achieve. The change management method used is the so-called “Breakthrough Method” developed by the Institute for Healthcare Improvement in Boston. This method, also called “Plan-Do-Study-Act”, is known all over the world and is used in quality improvement projects. It is based on the identification of measurable objectives, followed by planning, implementation, observation with evaluation (measurement) and then the implementation of any adjustments (4,5).
Another important aspect is the financial sustainability of these projects. This problem can justify the scepticism of the leadership of health care provider organizations. In fact, especially in the beginning, the ERAS protocols may seem expensive; however in a medium time perspective, the relevant economic allocation can result in a return in terms of savings and therefore in the possibility of further development plans: the “return on investment” (ROI) concept. Two other barriers have to be overcome for the implementation of ERAS. The first one is related to staff: it is sometimes very difficult to involve in a changing process professionals who have worked in a certain way for many years and maybe since the beginning of their working life. The other barrier is patient-related: patients in a difficult moment of their life, like a recent diagnosis of tumor, may have the impression that they are undergoing some kind of experimental procedures. All this, is clearly correlated with patients’ cultural level, their ability to fully understand the proposed therapeutic program and therefore the ability to adhere to it (5).
As far as thoracic surgery is concerned no clear ERAS guidelines are published yet and only few of the papers regarding ERAS are useful in order to point out all the aspects of the ERAS thoracic surgery project (6-8).
In a recently published paper, Jurt et al. (9) clearly demonstrated that better adherence to the protocol is associated to a shorter hospital stay and a decreased complication rate. In particular a statistical significant difference is evident between patient who had less than 70% adherence and patients that had more than 80% adherence.
Unfortunately sometimes surgeons are too much worried about some items which are not that much important for patients. Only a handful of studies report patients’ perspective. In a qualitative patient-led study Gillis et al. (10) showed what really is important for the patients: they want to be better informed during the preoperative counselling about what ERAS really is and what they are asked to do. They also would like to have more comprehensive discharge information. On the contrary writing a daily journal, a very important thing for the doctors, was not considered really important by the patients.
In the last few years many different centres have focused on ERAS programs so too many protocols are now available and there are too many elements to be considered. Identifying which items are really important could really be helpful. In a paper published in 2016 on 614 patients Thorn et al. (11) define active and passive items. Passive items are all of the elements that do not require patient’s will to be carried out such as thoracic epidural anaesthesia, intraoperative fluid treatment or avoidance of nasogastric tube and urinary catheter.
Active items meanwhile definitely need patients’ cooperation to be carried out; for example they include switch to oral analgesia and early mobilization.
Passive compliance reported in the study was quite high (93.6%) especially if compared to active compliance that was only 56.5%.
Furthermore what is really important is that poor active compliance is associated with increased major morbidity and increased length of stay so it has a strong predictive value for surgical outcomes.
As active elements are mostly found in the postoperative phase there should be a strong emphasis on the need of a postoperative counselling to the patients; surgeons usually tend to give a lot of information about preoperative and intraoperative procedures forgetting the postoperative phase. This may lead the patients to what is described by Nicolescu et al. (12) as post-hospital syndrome. The ERAS program should not be limited to the perioperative period, but should include the journey from diagnosis to complete patient recovery.
Patient education is of paramount importance when speaking of ERAS, in fact the best surgical outcomes are possible only when patients take ownership and responsibility for their role; only by doing this the patients can fully understand the different items of the protocol and adhere to them in the best way (6).
Regarding patients’ attitude to collaborate with doctors in an ERAS program very interesting work comes from Graffigna et al. (13,14). In her paper the author clearly demonstrates that PAM 13 (Patient Activation Measure), with its Italian translation PAM 13-I is a valid and reliable instrument to assess patients’ ability to deal with disease. A high patient activation, which is described as “..the knowledge, skills, confidence and behaviours for managing one’s own health and health care…” (15), leads to better management of the disease and better adherence to the protocol which eventually translates in better clinical outcomes.
In conclusion ERAS is a patient-tailored process, every patient has its own experience and patients’ feedback is of paramount importance to build a successful program. ERAS is not a single and rigid protocol but is a method, a “modus operandi”, a new way of multidisciplinary teamwork with readiness to make changes as knowledge evolves, i.e., a revolution of medical-scientific thought, we have to move from the concept of “management of disease” to that of “health promotion” (6).
The message that patients bring to ERAS is “…if you tell us why and help us understand what we need to do, we’ll be more than happy to do all we can…”. That is why patients have to be invited into ERAS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41. [Crossref] [PubMed]
- Senturk JC, Kristo G, Gold J, et al. The Development of Enhanced Recovery After Surgery Across Surgical Specialties. J Laparoendosc Adv Surg Tech A 2017;27:863-70. [Crossref] [PubMed]
- Ljungqvist O, Young-Fadok T, Demartines N. The History of Enhanced Recovery After Surgery and the ERAS Society. J Laparoendosc Adv Surg Tech A 2017;27:860-2. [Crossref] [PubMed]
- Roulin D, Najjar P, Demartines N. Enhanced Recovery After Surgery Implementation: From Planning to Success. J Laparoendosc Adv Surg Tech A 2017;27:876-9. [Crossref] [PubMed]
- Schatz C. Enhanced Recovery in a Minimally Invasive Thoracic Surgery Program. AORN J 2015;102:482-92. [Crossref] [PubMed]
- Che G. Establishment and Optimization of Enhanced Recovery after Surgery System for Lung Cancer. Zhongguo Fei Ai Za Zhi 2017;20:795-9. [PubMed]
- Li S, Zhou K, Che G, et al. Enhanced recovery programs in lung cancer surgery: systematic review and meta-analysis of randomized controlled trials. Cancer Manag Res 2017;9:657-70. [Crossref] [PubMed]
- Jurt J, Slieker J, Frauche P, et al. Enhanced Recovery After Surgery: Can We Rely on the Key Factors or Do We Need the Bel Ensemble? World J Surg 2017;41:2464-70. [Crossref] [PubMed]
- Gillis C, Gill M, Marlett N, et al. Patients as partners in Enhanced Recovery After Surgery: A qualitative patient-led study. BMJ Open 2017;7:e017002. [Crossref] [PubMed]
- Thorn CC, White I, Burch J, et al. Active and passive compliance in an enhanced recovery programme. Int J Colorectal Dis 2016;31:1329-39. [Crossref] [PubMed]
- Nicolescu TO. Perioperative Surgical Home. Meeting tomorrow's challenges. Rom J Anaesth Intensive Care 2016;23:141-7. [PubMed]
- Graffigna G, Barello S, Bonanomi A. The role of Patient Health Engagement Model (PHE-model) in affecting patient activation and medication adherence: A structural equation model. Available online: https://doi.org/ [Crossref]
- Graffigna G, Barello S, Bonanomi A, et al. Measuring patient activation in Italy: translation, adaptation and validation of the Italian version of the patient activation measure 13 (PAM13-I). BMC Medical Informatics and Decision Making 2015;15:109. [Crossref] [PubMed]
- Hibbard JH, Greene J, Tusler M. Improving the outcomes of disease management by tailoring care to the patient's level of activation. Am J Manag Care 2009;15:353-60. [PubMed]
Cite this article as: Taurchini M, Del Naja C, Tancredi A. Enhanced Recovery After Surgery: a patient centered process. J Vis Surg 2018;4:40.


