Endobronchial ultrasound-guided transbronchial needle aspiration for staging of non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death in the United States (1). The majority of lung cancers are non-small cell and 85% of patients have locally advanced disease at the time of diagnosis. Accurate preoperative staging of non-small cell lung cancer (NSCLC) is important as it informs treatment options. Imaging is a mainstay of cancer staging. Computed tomography (CT) and positron emission tomography (PET) scans can identify lymph nodes suspicious for metastases; however, histologic confirmation is required (2,3). Minimally invasive techniques for lymph node biopsy, such as endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), have gained popularity due to their ability to accurately stage for patients while minimizing pain and complications associated with more invasive procedures, such as mediastinoscopy and thoracoscopy. By combining conventional bronchoscopic techniques and ultrasound imaging, EBUS-TBNA provides surgeons with a novel method of tissue sampling, involving the use of a fine-gauge needle, that is associated with reduced morbidity compared to mediastinoscopy, the current gold-standard for mediastinal lymph node staging (4-6). In addition to having a reported sensitivity in identifying nodal metastases in the setting of lung cancer ranging from 90% to 98%, EBUS-TBNA is advantageous because it is a safe and cost-effective procedure that can be used in patients who are poor surgical candidates or who have pathological processes not requiring surgical intervention (e.g., sarcoidosis, lymphoma) (2,5-8).
Despite its popularity and advantages for clinicians and pathologists, EBUS-TBNA has some challenges. Variation in needle aspiration yields may be related to multiple factors, including surgeon expertise in EBUS-TBNA and differences in size, location, and cellularity of the target lymph nodes (9). However, biopsy potential may be maximized with improvement of TBNA skill, as endobronchial ultrasonography merely provides visualization of target tissue (10). Other limitations include limited field of view from the camera and limited flexibility of the bronchoscope that make airway intubation and inspection of lymph nodes more difficult than mediastinoscopy; these challenges can be overcome with additional training in the EBUS-TBNA technique (6).
EBUS-TBNA provides access to several lymph node stations that are otherwise difficult or impossible to access with conventional mediastinoscopy. Accessible levels include 1 (highest mediastinal), 2R and 2L (upper paratracheal), 4R and 4L (lower paratracheal), and 7 (subcarinal) mediastinal lymph nodes. Hilar lymph nodes accessible by EBUS-TBNA include levels 10 (hilar), 11 (interlobar), and 12 (lobar) (9). This technique is useful for lung cancer staging or restaging after neoadjuvant chemotherapy, especially if the patient had a mediastinoscopy prior to neoadjuvant therapy.
Patient selection and workup
Indications for EBUS-TBNA are similar to those for cervical mediastinoscopy. Chest CT with contrast and PET scan provide visualization of lymph nodes in relation to vascular structures and the airway. Enlarged or FDG-avid mediastinal and hilar lymph nodes or mass lesions warrant histological evaluation via EBUS-TBNA.
Thorough analysis of all lymph nodes allows for strategic planning of the order for biopsy of different nodal stations. For patients with potential or known malignancy, sampling of the node that would stage the disease at its highest level is recommended to preclude unnecessary further invasive surgical staging (9).
In addition to reviewing lymph node stations, the locations of major vascular structures should be reviewed prior to commencement of EBUS-TBNA. Particular attention should be paid to the aortic arch in relation to the left hilar structures, the right main pulmonary artery in relation to the trachea and carina, the azygos vein and superior vena cava in relation to the right paratracheal region, and the branching pulmonary arteries in relation to the branching airways (9).
Our patient is an 81-year-old man with a history of hypertension, abdominal aortic aneurysm, peripheral vascular disease and osteoarthritis who initially presented to his primary care provider with a productive cough and was found to have a nodular opacity in the left lower lobe on chest radiograph. Upon follow up, his symptoms had resolved, but repeat chest radiograph demonstrated incomplete resolution of the left lower lobe nodular opacity and chest CT was recommended. His performance status (Zubrod score) was 0.
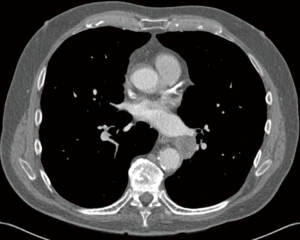
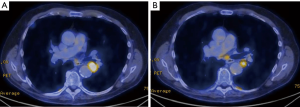
Chest CT demonstrated a heterogeneous, well-circumscribed central left lower lobe mass measuring 2.4 cm × 2.5 cm (Figure 1). PET scan showed a hypermetabolic mass in the left lower lobe with standardized uptake value (SUV) of 17.5, concerning for primary lung cancer (Figure 2A). A hypermetabolic subcarinal lymph node was also noted with an SUV of 3.9 (Figure 2B). Incidentally, extensive emphysematous changes to the bilateral lungs were also identified. Magnetic resonance imaging (MRI) of the brain demonstrated no intracranial metastatic disease.
Given the importance of tissue diagnosis and staging to guide treatment options, the patient elected to undergo flexible bronchoscopy, EBUS-TBNA of the primary lesion and mediastinal staging.
Preoperative preparation
EBUS-TBNA may be performed via total intravenous anesthesia (TIVA) or volatile gas anesthesia (11). TIVA is preferred over the use of volatile anesthetic agents because it prevents contamination of the procedure room atmosphere with volatile anesthetic due to frequent access of the airway by the surgeon. Additionally, frequent suctioning of the airway may result in inconsistent delivery of volatile anesthetic to the patient. Finally, the risk of increased bleeding at biopsy sites, believed to be associated with vasodilation of bronchial and pulmonary vasculature caused by volatile anesthetic agents, makes TIVA the preferred method of anesthesia delivery (11).
Unlike mediastinoscopy, EBUS requires the anesthesiologist to share the airway with the surgeon performing the EBUS-TBNA procedure. The anesthesiologist may support the patient’s pulmonary function via laryngeal mask ventilation or endotracheal intubation. Laryngeal mask ventilation is advantageous because it allows the surgeon to assess and biopsy high paratracheal lymph nodes that are otherwise difficult to access with an endotracheal tube in place. Increasing airway pressures produced by the tight-fitting endotracheal tube and EBUS scope also makes patient ventilation difficult; whereas laryngeal mask ventilation provides adequate ventilation around the bronchoscope (5,11).
Equipment preference card
The EBUS system consists of the EBUS bronchoscope with video processor and light source and an ultrasound processor, capable of ultrasound and Doppler flow analysis, to provide real-time imaging. Other required equipment includes:
- Monitors;
- Saline;
- Syringes;
- Biopsy needles (ranging in size from 19 to 22 gauge with a length ranging from 13 to 15 mm).
Procedure
Prior to EBUS-TBNA, a thorough examination of the airway is performed using standard bright light bronchoscopy for inspection of the tracheal and endobronchial anatomy, clearance of secretions and to identify endobronchial lesions. A thorough ultrasound investigation of the mediastinum is performed with the EBUS, providing practical information about the location and accessibility of lymph node targets delineated by preoperative imaging. A disposable latex balloon is fitted to the tip of the ultrasound probe and inflated with saline to enhance contact of the probe with the wall of the airway and optimize the ultrasound signal.
Lymph nodes
Ultrasound evaluation of lymph nodes is performed using the EBUS scope. Typically, lymph nodes are characterized as having a grainy, homogenous echotexture with a hyperechoic capsule. However, lymph nodes greater than 1 cm in diameter, having a round morphology with sharply defined margins, or hypogenic areas typical of cystic degeneration or necrosis warrant concern (5).
Biopsy
After identifying target lymph nodes and in preparation for biopsy, the EBUS scope should be aligned with the target tissue as the diagnostic advantage of TBNA is increased when the EBUS probe is adjacent to the lesion (12). A biopsy needle, typically 21 or 22 gauge, is inserted through the working channel and locked into place. The bronchoscope should be in neutral position while inserting the needle to avoid damage to the bronchoscope. Color flow Doppler imaging is used to ensure that the needle does not puncture vascular structures. Next, the plastic sheath is advanced until it is visible on the bronchoscopic view. With the sheath in proper position, the needle will not injure the working channel of the bronchoscope while performing the biopsy. The surgeon determines how deep the needle needs to be inserted to biopsy the lymph node and sets the depth on the scope. The biopsy needle is deployed rapidly through the bronchial wall and into the target lymph node via a jabbing motion as an assistant stabilizes both the bronchoscope and the endotracheal tube or LMA. If the needle is deployed too slowly, the scope may be pushed away from the bronchial wall. If this occurs, visualization will be obscured and it can be mitigated by slowly advancing the bronchoscope until the original view is obtained. Additionally, if the needle is met with resistance due to the cartilaginous bronchial wall, the scope may be adjusted to a “soft spot” and the needle redeployed rapidly (5).
After needle insertion into the target tissue is complete, either capillary action or suction can be used to draw cells up into the needle. As the surgeon is repeatedly passing the needle into a lymph node, an assistant slowly removes the stylet. Alternatively, suction can be applied. In the event of bloody return, suction should be removed immediately, the needle should be retracted and removed from the working channel. In general, minimal bleeding occurs and stops spontaneously; another attempt can be made at a different site after bleeding stops (9).
The first pass should be performed with a small gauge needle, such as 22 gauge, to minimize the risk of bleeding. When the safety of the puncture site is established, a larger bore needle, 19 gauge, can be used to obtain specimen providing adequate tissue samples for pathologic analysis of tissue architecture (9) if needed.
To obtain an FNA biopsy, the bronchoscope should be fixed in place while a rapid and shallow in-and-out motion of the needle is performed. Attention should be paid to keeping the scope straight; as much of the jabbing motion may be lost to slack in the scope before reaching the needle. It is recommended that the needle is passed 15–20 times into the target lymph node for adequate sampling of tissue. To ensure that the needle remains within the lymph node, ultrasound monitoring of the needle tip is performed. Before pulling the needle back into the catheter, suction (if used) is released, minimizing the aspiration of bronchial cells with the biopsy specimen. To minimize contamination, a different aspiration needle should be used at each biopsy site.
Although EBUS-TBNA is a popular sampling technique, details of sampling methodology, such as the number of aspirations per target and the need for rapid on-site cytological evaluation (ROSE), varies in the surgical literature. A recent study reported that, when ROSE was unavailable, maximal diagnostic values for EBUS-TBNA was achieved with three aspirates (5,8). However, judgements concerning the number of aspirates should be made according to macroscopic appearance of the target tissue (8). ROSE is advantageous because it allows for immediate evaluation of the biopsy sample for adequacy. ROSE has been shown to be both cost-effective and has improved diagnostic yield (5,8,9).
Role of team members
As with all surgical procedures, a team approach is required to maximize efficiency in the operating room. The surgical team consists of:
- Surgeon and assistant;
- Anesthesiologist;
- Circulating nurse;
- Scrub nurse;
- Pathology team.
The surgeon is the leader and directs team members to improve coordination of care in the operating room. It is fundamental that the surgeon is trained in the EBUS-TBNA technique and is versed in the principles of mediastinoscopy. The surgeon should also be prepared to guide the team in addressing unanticipated intraoperative events and complications.
The anesthesiologist is a key member of the team responsible for monitoring and maintaining the patient’s hemodynamic and pulmonary stability. The anesthesiologist will perform either total intravenous or volatile gas anesthesia and maintain analgesia throughout the operation and ensure that the airway remains secure.
In addition to providing tissue diagnosis, pathologists and cytotechnologists offer ROSE to evaluate the cellular content of the biopsy specimens and determine adequacy of fine-needle aspiration smears and touch imprints (13). This service may also reduce costs and morbidity because it requires fewer needle passes and minimizes the need to repeat a procedure due to inadequate sampling (14). Lastly, ROSE allows pathologists to triage samples to appropriate ancillary tests including: immunohistochemistry, flow cytometry, and culture (15). The efficiency and cost of ROSE may be improved with telepathology: the transmission of static images, video microscopy, or whole scanned slides to the remote pathologist for review (16).
Postoperative management
Complications after EBUS-TBNA are rare and occur in less than 1% of cases; they include endobronchial bleeding, mucosal laceration, and infection after EBUS-TBNA (5). Additionally, patients may experience bronchospasm and laryngospasm caused by irritation of the airway from multiple passes of the bronchoscope through the vocal cords and into the airway; mild postoperative cough and sore throat often resolve within 8 hours and require no further intervention (11).
Our patient was extubated in the operating room and transferred to the post-anesthesia care unit for monitoring. He required no analgesics and was discharged home the same day. Histopathologic evaluation of the left lower lobe and lymph node biopsy specimens demonstrated squamous cell carcinoma. This case was presented in multidisciplinary Tumor Board to discuss therapeutic options including: definitive chemoradiation therapy and induction therapy followed by surgery. Given the central location of the mass, clear surgical margins would be difficult to obtain and his pulmonary function did not allow for lobectomy. The recommendation was definitive chemoradiation therapy.
We present a patient who underwent endobronchial ultrasound transbronchial needle aspiration and detail the technical aspects of the procedure (Figure 3).

Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this study and any accompanying images.
References
- French CA. Respiratory Tract and Mediastinum. In: Cibas ES, Ducatman BS. editors. Cytology: Diagnostic Principles and Clinical Correlates. 4 ed. Philadelphia, PA: Elsevier, 2014:59-104.
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of Mediastinal Adenopathy—Real-Time Endobronchial Ultrasound Guided Needle Aspiration versus Mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [Crossref] [PubMed]
- Crabtree TD. Endobronchial Ultrasound. Oper Tech Thorac Cardiovasc Surg 2009;14:99-111. [Crossref]
- Monaco SE, Khalbuss WE, Pantanowitz L. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA): A Practical Approach. Karger, 2014.
- Vincent BD, El-Bayoumi E, Hoffman B, et al. Real-time endobronchial ultrasound-guided transbronchial lymph node aspiration. Ann Thorac Surg 2008;85:224-30. [Crossref] [PubMed]
- Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [Crossref] [PubMed]
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [Crossref] [PubMed]
- Kurimoto N, Fielding DI, Musani AI. Endobronchial Ultrasonography. Oxford, UK: Wiley-Blackwell, 2011.
- Hsu LH, Liu CC, Ko JS. Education and experience improve the performance of transbronchial needle aspiration: a learning curve at a cancer center. Chest 2004;125:532-40. [Crossref] [PubMed]
- Sarkiss M, Kennedy M, Riedel B, et al. Anesthesia technique for endobronchial ultrasound-guided fine needle aspiration of mediastinal lymph node. J Cardiothorac Vasc Anesth 2007;21:892-6. [Crossref] [PubMed]
- Chao TY CM, Lie CH. Endobronchial Ultrasonography-Guided Transbronchial Needle Aspiration Increases the Diagnostic Yield of Peripheral Pulmonary Lesions. Chest 2009;136:229-36. [Crossref] [PubMed]
- Harnish B, Nidhi V, Neena D. Usefulness of touch Imprint Cytology in Cancer diagnosis: A study of 119 cases. International Research Journal of Medical Sciences 2014;2:19-25.
- Mallya V, Kumar S, Meganathan P, et al. The utility of ROSE (rapid on-site evaluation) in endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA): Is the picture rosy? J Cytol 2015;32:230-3. [Crossref] [PubMed]
- Schmidt R, Walker BS, Cohen MB. When is Rapid On-Site Evaluation Cost-Effective for Fine-Needle Aspiration Biopsy. PLoS One 2015;10:e0135466. [Crossref] [PubMed]
- Tambouret RH, Barkan GA, Kurtycz DFI, et al. Cytopathology and More. FNA cytology: Rapid on-site evaluation—how practice varies. CAP TODAY 2014.
- Hashimi H, Cooke DT, David EA, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for squamous cell carcinoma. Asvide 2018;5:094. http://asvidett.amegroups.com/article/view/22987
Cite this article as: Hashimi H, Cooke DT, David EA, Brown LM. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of non-small cell lung cancer. J Vis Surg 2018;4:37.


