State-of the-art review on the renal and visceral protection during open thoracoabdominal aortic aneurysm repair
Introduction
Postoperative acute kidney injury (AKI) is a common complication after open thoracoabdominal aortic aneurysm (TAAA) repair (OTAAAR) with an incidence that ranges between 21% and 63%, depending on the definition of AKI (1-3). About 2.7% to 10.7% of the patients will develop kidney failure requiring temporary or permanent dialysis (4-7). This percentage is even higher in case of emergent surgery or in case of chronic kidney disease (CKD) (8,9). Beside reduced quality of life (QoL) and longer hospital stay, the early mortality after OTAAAR rises significantly from 5–19% to 8.9–32% in case of AKI and can be as high as 63% when postoperative dialysis is needed (10-13). Furthermore, the 5-year survival after OTAAAR decreases from 74% to 43% in case of postoperative CKD (1). Although visceral complications after OTAAAR have a lower incidence of around 7%, they also have been associated with significant morbidity and mortality rates of 15% to 63% (14-17).
As Crawford type II, III, and IV TAAAs require the reattachment of the renal and visceral [celiac trunk and superior mesenteric artery (SMA)] arteries during repair, there is an inevitable period of renal and visceral ischemic time, regardless of the surgical technique. Distal aortic retrograde perfusion systems are the most common used methods for organ protection during OTAAAR (11,18). However, debates on the optimal technique for organ preservation are ongoing, especially regarding perioperative renal and visceral preservation (19-21). Various renal and visceral protective techniques have been reported, such as SRP and SVP, DAP, hypothermic circulatory arrest, and cold perfusion of the renal arteries after aortic clamping (2,14,22-27). Specialized centers reported reduced perioperative mortality rates of <10% by following these surgical strategies (5,28,29). However, the persistent high renal and visceral complication rates indicate that these protection measures remain suboptimal and require optimization. Furthermore, patient selection plays an important role in predicting the outcome after OTAAAR (9,30). Pre-existing renal disease is the most important risk factor for developing postoperative AKI (10,31,32). Other risk factors that are associated with renal and/or visceral complications after OTAAAR include advanced age, the duration of renal and visceral ischemia, prolonged intraoperative hypotension, large surgical blood loss, associated atherosclerotic arterial vessel disease, history of cerebrovascular disease, diabetes mellitus, and simple aortic clamp technique without antegrade or selective perfusion (7,8,10,22,30,33,34).
This state-of-the-art review gives an overview of the current and most evidence-based renal and visceral protection methods during OTAAAR, based on the most recent publications and personal experience.
Methods
An electronic search was performed in four medical databases: PubMed, Web of Science, Embase, and the Cochrane Library. The following MeSH terms were used: thoracoabdominal aneurysm, TAAAR, visceral protection, renal protection, kidney, perfusion, and intestines. Every trial, case report, review, and editorial was considered. As this review focuses on the newest protection techniques, the search for publications was limited to the last 10 years. There was no focus on publication language or impact factor of the journal. A total of 39 relevant publications were included in this review. To make sure that other relevant articles were not missed, the references in all found publications were additionally screened. The function “Related articles” was also used on a regular basis. The literature search was ended on August 31st, 2017.
Results
Cardiac bypass circuit
With the development of mechanical circulatory support systems, the classic approach of simple aortic cross clamping without any distal perfusion strategy has been abandoned completely. In large aortic surgery centres, the LHB is currently the most frequent approach to provide DAP during OTAAAR. Its implementation has been described by multiple authors (11,14,29,35,36). However, some studies have reported an increased incidence of AKI after the use of LHB, which is probably due to the direct cannulation of the left common femoral artery with subsequent risk of leg ischemia and myoglobin increase (37-39). An alternative arterial cannulation technique is using a short graft, which is sewn end-to-side onto the femoral artery. Miller et al. compared both cannulation methods and found a 15% to 20% reduction in postoperative AKI in patients with CKD, suggesting an advantage of this alternative cannulation technique in high-risk patients (40). When the iliac arteries are occluded and distal aortic retrograde perfusion is not feasible, Monnot et al. recently described the possibility of using a temporary arterial shunt inserted onto the left axillary artery to provide passive renal and visceral perfusion after the aorta is opened (18). They reported good outcomes in the first series of 10 patients.
Table 1 gives an overview of the possible inflow and outflow cannulation sites. Besides the LHB, full cardiopulmonary bypass (CPB) is another approach to provide DAP, but it is less frequently used. Additional organ protection can be provided by delivering some level of systemic hypothermia during cardiac bypass to prolong the ischemic tolerance during aortic occlusion. Both mild systemic hypothermia (30–34 °C), as well as deep hypothermic circulatory arrest (DHCA) (15–18 °C) are possible (41-44). However, several studies show that DHCA has a more protective effect in case of increased organ ischaemia time (over 60 minutes), while mild hypothermia is the better choice for shorter ischaemic times (45-50).
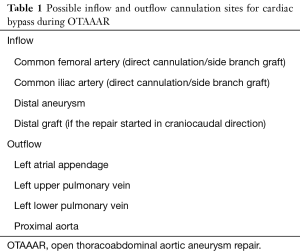
Full table
SRP strategies
Cold crystalloid solution has been the standard approach for SRP for the last two decades and several studies have reported excellent renal protection (2,8-10,51,52). During SRP, it is important to notice an increase in arterial pressure due to the volume added to the circulation and a substantial drop in systemic temperature. Although the usual target renal temperature throughout the ischemic period used to be aimed at 15 °C or less, more recent data suggested that temperatures between 15 and 28 °C provide excellent protection as well (3,39,53). Besides, an aimed kidney temperature of 15 °C or less is often not reachable within the constraints of avoiding fluid overload and severe systemic hypothermia. Although selective blood perfusion of the kidneys would seem like a logical choice to reduce the renal ischemic time during aortic clamping, Köksoy et al. reported a significantly higher incidence of postoperative AKI when using normothermic (37 °C) blood, compared to cold (4 °C) crystalloid perfusion (63% vs. 21%, P=0.03) for SRP (2). On the contrary, cold blood and cold crystalloid solution seem to be equally effective for SRP, with no significant differences in level of renal hypothermia, renal outcome, or mortality (3).
Custodiol is a well-known enriched and buffered crystalloid solution that is routinely used for cardioplegia in open heart surgery, as well as for the preservation of organs during transplant surgery. Although currently not routinely used for SRP, one study reports a significant lower rate of postoperative AKI (P=0.002) when using Custodiol, compared to cold crystalloid perfusate (34).
As to the mode of perfusion administration, the initial approach was to only assess the volume flow in each perfusion catheter (volume-controlled perfusion). Jacobs et al. later reported an added benefit of providing pressure-controlled selective perfusion for the renal and visceral arteries (6). A perfusion pressure of at least 60 mmHg is suggested and can be measured with pressure-sensitive catheter tips that are placed in the renal and visceral arteries. Analogous to the study by Miller et al., this was especially advantageous for patients with CKD, as none of the CKD-patients developed renal failure postoperatively when receiving adequate pressure-controlled renal perfusion. Jacobs et al. even suggested a mean selective perfusion pressure of 85 mmHg or more in case of CKD (6,40). In addition to a pressure- and volume-controlled renal perfusion, a recent study by Gallinat et al. states the importance of pulsatile machine perfusion of the renal arteries, which should result in lower tubular damage and higher creatinine clearance, compared to non-pulsatile (continuous flow) perfusion (54).
Adequate arterial permeability is of course crucial to obtain sufficient renal cooling. This is often a problem due to atherosclerotic plaques or dissecting membranes in case of chronic dissections. Therefore, preoperative optimization of the renal perfusion is an attractive goal. Sullivan et al. were among the first to report their successful experience with preoperative renal stenting in case of renal artery stenosis (55). Yue et al. reported successfully postoperative renal artery thrombectomy with normalisation of the renal function (56). These pre- and postoperative renal stenting reports stimulated research on perioperative renal stenting during OTAAAR. LeMaire et al. inserted 80 stents in either one or both renal arteries in 400 patients undergoing OTAAAR (57). No significant differences in morbidity or mortality were reported between the stented and non-stented groups. Although the stenting itself was a technical success, the need for postoperative haemodialysis was the highest in the group of patients undergoing stenting as an adjunct to renal endarterectomy.
SVP
Clearly, intestines can tolerate a much longer ischemic period than the kidneys before irreversible damage occurs. In contrary to SRP, SVP is not routinely used during OTAAAR. To evaluate the importance of SVP, Kalder et al. set up a porcine model (15 pigs) of thoracic aortic cross-clamping with or without SVP (58). They found that SVP resulted in a significantly better microcirculation (blood flow in the intestinal mucosal and muscular layers) (P=0.0018), less acidosis (P=0.0221), less extensive tissue damage, and less inflammation (IL-8 production) (P=0.0374). However, mucosal injury and lactate acidosis were still detectable in the SVP-group, proving that SVP remains insufficient for visceral protection. Regarding the effect of SVP on the hepatosplanchnic metabolism, Kunihara et al. found that patients who received SVP during OTAAAR did not develop coagulopathy, nor clinically relevant liver or renal dysfunction (42). However, they did note a significant decrease in hepatic venous oxygen saturation and lactate extraction ratio, suggesting that SVP cannot deliver physiologically adequate blood flow and that the duration of SVP should be minimised.
In a follow-up study in 2015, Kalder et al. investigated the effect of DAP on the perfusion of the intestinal wall (59). Using the same porcine model setup and adding DAP to both the SVP- and non-SVP-group, they found that DAP did indeed establish adequate flow rates and mean arterial blood pressures in the visceral arteries. They also suggested to use extracorporeal circulation (ECC) with normal flow rates, as this will result in a lower increase of lactate acidosis, compared to ECC with a low flow rate.
Perfusate additives
Commonly used crystalloid perfusion formulas are lactated Ringer’s solution (Hartmann) or 0.9% NaCl solution, with several additives including mannitol, methylprednisolone, and heparin (21,27,39). The added benefit of mannitol has been described extensively: it causes an expansion of the intravascular and extracellular fluid volumes, decreases blood viscosity, induces renal vasodilatation, reduced tubular cell swelling, and produces an osmotic diuresis. Moreover, mannitol is an oxygen free radical (OFR) scavenger and will eliminate the OFR’s, associated with ischaemia-reperfusion injury. The previously mentioned study by Tshomba et al. reports an even greater benefit when adding histidine-tryptophan-ketogluterate to the perfusate (34).
Additionally, a recent randomised controlled trial (RCT) by Mori et al. reported that a continuous infusion of atrial natriuretic peptide (ANP) during and after the operation could further diminish the incidence of postoperative AKI (60). ANP is known to dilate the renal arteries and increase the glomerular filtration rate (GFR), acting as a diuretic, natriuretic and anti-inflammatory renal agent. Previous studies have already shown that a low-dose ANP infusion reduced the need for haemodialysis in patients after cardiovascular surgery (61-63). In a group of 42 patients who received aortic arch surgery, Mori et al. showed that intraoperative ANP resulted in a significant lower incidence of AKI (P=0.014) and a significant higher perioperative urine output (P=0.005) (60). There was, however, no significant difference in the need for dialysis and 30-day mortality.
Reducing the organ ischemia time (IT)
Prolonged renal and visceral IT remains one of the most important risk factors for AKI and visceral ischemia after OTAAAR (7,10,22,30,33). Several factors can result in a prolonged IT, such as technical difficulties, severe atherosclerotic arterial vessel disease or remote location of the renal or arterial vessel. In case of the latter two, a bypass graft from the aortic prosthesis to the renal and/or visceral arteries is sometimes necessary. Although this has been described before with great feasibility and excellent graft patency, this added step can be time consuming (64). By using covered self-expanding stents and performing so called “sutureless anastomoses”, difficult vessel exposures and graft anastomoses can be avoided and the IT can be shortened. Lachat et al. first described this innovative technique in 2008 with the VORTEC stent graft (65). A covered stent graft was deployed into the renal artery and end-to-end sutured onto a bypass graft, which was in turn end-to-side sutured onto the main aortic graft. Excellent technical success and patency rates were reported. The main shortcoming of this technique was the need to still perform two anastomoses (stent to bypass graft and bypass graft to aortic graft). Chiesa et al. recently reported their first experiences with a more novel hybrid vascular graft, consisting of a vascular prosthesis that includes a nitinol-reinforced self-expanding section at one of its extremities, allowing a sutureless endovascular anastomosis with a renal or visceral vessel (33). The prosthesis itself can be sutured onto the aortic graft, thus eliminating one extra anastomosis per vessel. Possible indications for this hybrid graft were remote location of the vessels, renal artery stenosis, and poor quality of the surrounding aortic wall (in case of aortic dissection or severe atherosclerotic disease). Compared to a group of patients who received the standard anatomic repair, the graft-group had significantly shorter cold renal perfusion times. Although both the total renal ischemia times and rate of AKI were lower in the graft-group, these outcomes did not reach statistical significance.
Several other operative techniques have been reported through the years to further reduce the IT, such as creating a bevelled upper anastomosis to include the intestinal and right renal arteries, preserving visceral perfusion by placing a Javid shunt between the descending thoracic aorta and SMA after opening the aneurysma, and using a Y-shaped arterial line in case of LHB (17,26,53,66-68).
Discussion
Perioperative mechanical improvements in renal and visceral perfusion and pharmacologic support of renal function have been explored in patients in need of major cardiovascular surgery, including OTAAAR. The traditional approach of simple aortic cross clamping without any distal perfusion has become obsolete and the use of some sort of bypass circuit is routinely used. Mechanical circulatory support has gained tremendous popularity in the last decade and both ECC and LHB can be used to maintain blood flow to the kidneys and viscera during the proximal portion of the aortic repair, although the latter is the most preferred option in most centres. At our centre in Sint Jan (Bruges, Belgium) we have been using the LHB with a centrifugal pump since 1987. We believe it maintains an adequate proximal and distal perfusion during the clamping phase and can reduce the visceral and renal ischemic time, a theory that has been confirmed by Svensson et al. in 1993 (11). We also believe in the benefits of using a side branch graft on the common femoral artery and routinely apply it in case of CKD or when both iliac arteries are included in the aneurysm (40). A RCT would be useful to determine the benefits of this technique in low-risk patients.
To further reduce the organ IT and maximise visceral and renal blood perfusion, we opt for sequential aortic clamping during the repair phase: after placement of the proximal clamp, we place the distal clamp in succession at the level of T6 for the proximal anastomosis, T12 for the anastomosis of the intercostal arteries, and infrarenal for the anastomosis of the visceral and renal arteries. We truly believe that the use of a bypass circuit, in combination with sequential clamping and replacing the aorta in a cranio-caudal direction offers maximal benefit with regards to the reduction of IT. It is furthermore important to strive for adequate arterial pressure before, during and after aortic clamping to maximize end-organ perfusion and oxygen delivery. During aortic clamping, we strive for a proximal systolic pressure of ≥120 mmHg and a distal mean arterial pressure (MAP) of ≥100 mmHg.
In the line of other authors, we tend to perform an endarterectomy and/or stenting of the renal and/or visceral arteries in case of severe atherosclerotic disease or dissection (26,39). Despite the disadvantage that this will prolong the IT, it has been proven that renal endarterectomy is associated with significantly less renal failure (39,69). A hybrid vascular graft seems like an attractive solution, as it decreases the technical complexity and duration of the distal anastomosis in case of stenotic or dissected arteries (26). The technical feasibility and reported early patency rates are already promising (33,70). The concept of sutureless anastomoses can also be useful in patients with a connective tissue disease (such as Marfan syndrome) in which the remaining aortic tissue should be kept to a minimum to prevent aneurysmal formation of the aortic patch. In that case, separate vessel reattachments are preferable, which can be time consuming.
The incidence of postoperative AKI and visceral complications remains substantial and the rate of postoperative renal complications has not decreased in the last two decades, regardless of all the medical and surgical advances in protective strategies (71,72). The major causes of perioperative organ damage are the temporary ischemia and subsequent ischemia-reperfusion injury, arterial embolism, and stenosis or dissection of the reattached renal vessel (6,11,73,74). Multiple RCT’s have been conducted to compare different perfusate solutions and determine which one provided the best organ protection during the ischemic period (2,3,8,34,36,75,76). Cold perfusates generally provide the best protection and experimental studies have shown a significant reduction in renal oxygen consumption with cooling of the renal parenchyma (2,77-79). Based on Level B evidence, the 2010 guidelines recommend either the use of cold blood or cold crystalloid perfusion for renal protection during OTAAAR (76). In the spirit of those guidelines, the application of renal hypothermia has been reported by several authors (2,3,36,52,53,69,80,81). However, the debate between cold blood and crystalloid solution is still ongoing, as LeMaire et al. found no significant differences in renal outcome between both perfusates (3). Svensson et al. analysed Crawford’s series of patients and reports better outcomes for cold crystalloid solution in case of renal artery occlusive disease or when the aortic clamp time exceeds 30–45 minutes (11,39,69). We, as most authors, prefer the use of cold crystalloid renal perfusion, which is somewhat less cumbersome than the cold blood technique. However, one should be aware of the possible risk of volume overload and systemic hypothermia when cold crystalloid perfusion is being used. In addition to renal hypothermia, we opt for mild systemic hypothermia, which has proven to induce less systemic inflammatory response and less reperfusion organ injury, when the IT is less than 60 minutes (46,47,49,50). Tshomba et al. see benefits in using Custodiol over Ringers’s solution for SRP with a statistical evidence of reduced AKI (34). However, the analysis of the Ringer’s group was retrospective in nature, which makes comparing both groups difficult and subjective to bias. We mostly stand critical towards the systemic effects of Custodiol when using it in conjunction with LHB and prefer not to use it for SRP for now. We look forward to the results of a planned RCT, comparing both perfusates.
Overall, our selective perfusion protocol is as follows: SRP (1,000 mL Hartmann solution with 54 mL mannitol 15% (8 gr) at a temperature of 4 °C) is administered through perfusion/occlusion catheters at a rate of 100 mL/kidney/min and repeated every 20 minutes. The first dose is 300 mL/kidney, the following doses 200 mL/kidney. Similar techniques have been described elsewhere (9,21). The perfusion is currently delivered as a continuous flow (using a roller pump), but we are considering the possibility of applying pulsatile flow, as suggested by Gallinat et al. (54). In contrast to some other reports, we do not add methylprednisolone to the Ringer’s solution and clinical studies fail to show a clear benefit for the use of corticosteroids in reducing AKI (82,83). On the other hand, mannitol has been shown to reduce cell swelling and improve renal blood flow after ischemia (84). We have no experience in the use of ANP, but the short-term outcomes of Mori et al. seem promising (60).
We currently do not apply SVP during aortic clamping and previous studies have confirmed that it remains insufficient for visceral protection (42,58,85). A possible explanation could be that the flow and perfusion pressure during SVP remains insufficient to compensate for the SVP-induced hypoperfusion and, therefore, low-flow ischemia and mucosal injury still occurs (58). However, the debate on the use of visceral perfusion and shunting is ongoing and some authors continue to use this technique (26,42,66,67). Although it may help prevent some visceral complications, the possible benefit of SVP is strongly dependent on numerous factors, such as the systemic arterial pressure, oxygenation from the native (right) lung, visceral vascular resistance, and diameter of the shunt catheters used. Current innovations in catheters, ECC flow types, mechanical components, and pharmaceutical protection measures could increase the benefits of SVP in the future (33,86-89).
We currently believe that the best way to reduce visceral injury is simply by minimizing the IT. Additionally, DAP has proven to be effective in maintaining visceral arterial flow, which is why we strongly advise to install a cardiac bypass circuit to maintain DAP for as long as possible (59). Furthermore, it is important to carefully examine the preoperative scan and evaluate the wall of the lower part of the descending aorta. In case of mural thrombi, plain supraceliac clamping should be used with caution to prevent cholesterol emboli to the visceral arteries.
Additionally, we administer SDD preoperatively, as this will reduce the bacterial burden in the small intestine and thus limit bacterial translocation, caused by ischemic vascular injury (90). SDD is a well-known treatment in critically ill or septic patients and has proven to be one of the most evidence-based interventions in the reduction of mortality after sepsis (91). It involves the administration of parenteral and enteral antibiotics to eradicate potentially pathogenic aerobic gram-negative bacteria in the oropharynx and intestines (91,92). There are currently no studies that discuss the benefit of SDD before OTAAAR, but multiple RCT’s and meta-analyses have shown that SDD significantly reduces mortality, lower airway infections, blood stream infections, and controls antibiotic resistance in case of sepsis (91). We therefore believe that performing SDD in a preoperative setting can further prevent postoperative infections. Although there is no agreed-upon protocol of which antibiotics are most effective, our standard is to administer a combination of Ciproxine and Fluconazol 3 to 5 days prior to surgery until the day of surgery.
Conclusions
In order to reduce postoperative mortality and optimise surgical results, the preservation of renal and intestinal function is mandatory during and after OTAAAR. This requires not only optimal preparation with the avoidance of nephrotoxic drugs and SDD the days before planned surgery, but also intraoperative strategies such as strict limitation of renal and intestinal ischemia, systemic cooling, avoidance of hemodynamic instability (mainly anaemia, hypovolemia, and hypotension), and regional protective perfusion of the kidneys. Only by adopting a multi-modality approach aimed at all these topics, we will be able to further reduce these dramatic, and often deadly, complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nathan DP, Brinster CJ, Woo EY, et al. Predictors of early and late mortality following open extent IV thoracoabdominal aortic aneurysm repair in a large contemporary single-center experience. J Vasc Surg 2011;53:299-306. [Crossref] [PubMed]
- Köksoy C, LeMaire SA, Curling PE, et al. Renal perfusion during thoracoabdominal aortic operations: Cold crystalloid is superior to normothermic blood. Ann Thorac Surg 2002;73:730-8. [Crossref] [PubMed]
- Lemaire SA, Jones MM, Conklin LD, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:11-9. [Crossref] [PubMed]
- Bensley RP, Curran T, Hurks R, et al. Open repair of intact thoracoabdominal aortic aneurysms in the American College of Surgeons National Surgical Quality Improvement Program. J Vasc Surg 2013;58:894-900. [Crossref] [PubMed]
- Coselli JS, Bozinovski J, LeMaire S. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4. [Crossref] [PubMed]
- Jacobs MJ, Eijsman L, Meylaerts SA, et al. Reduced renal failure following thoracoabdominal aortic aneurysm repair by selective perfusion. Eur J Cardiothorac Surg 1998;14:201-5. [Crossref] [PubMed]
- Kashyap VS, Cambria RP, Davison JK, et al. Renal failure after thoracoabdominal aortic surgery. J Vasc Surg 1997;26:949-55; discussion 955-7. [Crossref] [PubMed]
- Wynn MM, Acher C, Marks E, et al. Postoperative renal failure in thoracoabdominal aortic aneurysm repair with simple cross-clamp technique and 4 °C renal perfusion. J Vasc Surg 2015;61:611-22. [Crossref] [PubMed]
- Girardi LN, Ohmes LB, Lau C, et al. Open repair of descending thoracic and thoracoabdominal aortic aneurysms in patients with preoperative renal failure. Eur J Cardiothorac Surg 2017;51:971-7. [Crossref] [PubMed]
- Schepens MA, Defauw JJ, Hamerlijnck RP, et al. Risk assessment of acute renal failure after thoracoabdominal aortic aneurysm surgery. Ann Surg 1994;219:400-7. [Crossref] [PubMed]
- Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg 1993;17:357-68; discussion 368-70. [Crossref] [PubMed]
- Kuniyoshi Y, Koja K, Miyagi K, et al. Selective visceral perfusion during thoracoabdominal aortic aneurysm repair. Ann Thorac Cardiovasc Surg. 2004;10:367-72. [PubMed]
- Chertow GM, Burdick E, Honour M, et al. Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients. J Am Soc Nephrol 2005;16:3365-70. [Crossref] [PubMed]
- Wong DR, Parenti JL, Green SY, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: Contemporary outcomes in 509 patients. J Am Coll Surg 2011;212:569-79. [Crossref] [PubMed]
- Achouh PE, Madsen K, Miller CC, et al. Gastrointestinal complications after descending thoracic and thoracoabdominal aortic repairs: A 14-year experience. J Vasc Surg 2006;44:442-6. [Crossref] [PubMed]
- Caty MG, Guice KS, Oldham KT, et al. Evidence for tumor necrosis factor-induced pulmonary microvascular injury after intestinal ischemia-reperfusion injury. Ann Surg 1990;212:694-700. [Crossref] [PubMed]
- Kieffer E, Chiche L, Godet G, et al. Type IV thoracoabdominal aneurysm repair: predictors of postoperative mortality, spinal cord injury, and acute intestinal ischemia. Ann Vasc Surg 2008;22:822-8. [Crossref] [PubMed]
- Monnot A, Dusseaux MM, Godier S, et al. Passive temporary visceral shunt from the axillar artery as an adjunct method during the open treatment of thoracoabdominal aortic aneurysm. Ann Vasc Surg 2016;36:127-31. [Crossref] [PubMed]
- Drews JD, Patel HJ, Williams DM, et al. The impact of acute renal failure on early and late outcomes after thoracic aortic endovascular repair. Ann Thorac Surg 2014;97:2027-33. [Crossref] [PubMed]
- Estrera AL, Jan A, Sandhu H, et al. Outcomes of open repair for chronic descending thoracic aortic dissection. Ann Thorac Surg 2015;99:786-93. [Crossref] [PubMed]
- Aftab M, Coselli JS. Renal and visceral protection in thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg 2014;148:2963-6. [Crossref] [PubMed]
- Safi HJ, Harlin SA, Miller CC, et al. Predictive factors for acute renal failure in thoracic and thoracoabdominal aortic aneurysm surgery. J Vasc Surg 1996;24:338-44; discussion 344-5. [Crossref] [PubMed]
- Frank SM, Parker SD, Rock P, et al. Moderate hypothermia, with partial bypass and segmental sequential repair for thoracoabdominal aortic aneurysm. J Vasc Surg 1994;19:687-97. [Crossref] [PubMed]
- Fehrenbacher JW, Hart DW, Huddleston E, et al. Optimal end-organ protection for thoracic and thoracoabdominal aortic aneurysm repair using deep hypothermic circulatory arrest. Ann Thorac Surg 2007;83:1041-6. [Crossref] [PubMed]
- Sheinbaum R, Ignacio C, Safi HJ, et al. Contemporary strategies to preserve renal function during cardiac and vascular surgery. Rev Cardiovasc Med 2003;4:S21-8. [PubMed]
- Coselli JS. Strategies for renal and visceral protection in thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg 2010;140:S147-9; discussion S185-90.
- Bhamidipati CM, Coselli JS, LeMaire SA. Perfusion techniques for renal protection during thoracoabdominal aortic surgery. J Extra Corpor Technol 2012;44:31-7. [PubMed]
- Mommertz G, Sigala F, Langer S, et al. Thoracoabdominal aortic aneurysm repair in patients with marfan syndrome. Eur J Vasc Endovasc Surg 2008;35:181-6. [Crossref] [PubMed]
- Schepens MA, Heijmen RH, Ranschaert W, et al. Thoracoabdominal aortic aneurysm repair: results of conventional open surgery. Eur J Vasc Endovasc Surg 2009;37:640-5. [Crossref] [PubMed]
- Schepens MA, Van den Brande FG. Patient selection for open thoracoabdominal aneurysm repair. Ann Cardiothorac Surg 2012;1:358-64. [PubMed]
- Huynh TT, Van Eps RG, Miller CC, et al. Glomerular filtration rate is superior to serum creatinine for prediction of mortality after thoracoabdominal aortic surgery. J Vasc Surg 2005;42:206-12. [Crossref] [PubMed]
- Acher C, Wynn M. Outcomes in open repair of the thoracic and thoracoabdominal aorta. J Vasc Surg 2010;52:3S-9S. [Crossref] [PubMed]
- Chiesa R, Kahlberg A, Mascia D, et al. Use of a novel hybrid vascular graft for sutureless revascularization of the renal arteries during open thoracoabdominal aortic aneurysm repair. J Vasc Surg 2014;60:622-30. [Crossref] [PubMed]
- Tshomba Y, Kahlberg A, Melissano G, et al. Comparison of renal perfusion solutions during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2014;59:623-33. [Crossref] [PubMed]
- LeMaire SA, Price MD, Green SY, et al. Results of open thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg 2012;1:286-92. [PubMed]
- Hassoun HT, Miller CC, Huynh TTT, et al. Cold visceral perfusion improves early survival in patients with acute renal failure after thoracoabdominal aortic aneurysm repair. J Vasc Surg 2004;39:506-12. [Crossref] [PubMed]
- Miller CC 3rd, Villa MA, Achouh P, et al. Intraoperative skeletal muscle ischemia contributes to risk of renal dysfunction following thoracoabdominal aortic repair. Eur J Cardiothorac Surg 2008;33:691-4. [Crossref] [PubMed]
- Miller CC, Villa MA, Sutton J, et al. Serum myoglobin and renal morbidity and mortality following thoracic and thoraco-abdominal aortic repair: does rhabdomyolysis play a role? Eur J Vasc Endovasc Surg 2009;37:388-94. [Crossref] [PubMed]
- Svensson LG, Coselli JS, Safi HJ, et al. Appraisal of adjuncts to prevent acute renal failure after surgery on the thoracic or thoracoabdominal aorta. J Vasc Surg 1989;10:230-9. [Crossref] [PubMed]
- Miller CC, Grimm JC, Estrera AL, et al. Postoperative renal function preservation with nonischemic femoral arterial cannulation for thoracoabdominal aortic repair. J Vasc Surg 2010;51:38-42. [Crossref] [PubMed]
- Fehrenbacher J, Siderys H, Shahriari A. Preservation of renal function utilizing hypothermic circulatory arrest in the treatment of distal thoracoabdominal aneurysms (types III and IV). Ann Vasc Surg 2007;21:204-7. [Crossref] [PubMed]
- Kunihara T, Shiiya N, Wakasa S, et al. Assessment of hepatosplanchnic pathophysiology during thoracoabdominal aortic aneurysm repair using visceral perfusion and shunt. Eur J Cardiothorac Surg 2009;35:677-83. [Crossref] [PubMed]
- Kulik A, Castner CF, Kouchoukos NT. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2011;141:953-60. [Crossref] [PubMed]
- Di Luozzo G. Visceral and spinal cord protection during thoracoabdominal aortic aneurysm repair: Clinical and laboratory update. J Thorac Cardiovasc Surg 2013;145:S135-8. [Crossref] [PubMed]
- Weiss AJ, Lin HM, Bischoff MS, et al. A propensity score-matched comparison of deep versus mild hypothermia during thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg 2012;143:186-93. [Crossref] [PubMed]
- Pacini D, Pantaleo A, di Marco L, et al. Visceral organ protection in aortic arch surgery: Safety of moderate hypothermia. Eur J Cardiothorac Surg 2014;46:438-43. [Crossref] [PubMed]
- Urbanski PP, Lenos A, Bougioukakis P, et al. Mild-to-moderate hypothermia for organ protection in aortic arch surgery with circulatory arrest: a change of paradigm? Eur J Cardiothorac Surg 2012;41:185-91. [PubMed]
- Khaladj N, Peterss S, Oetjen P, et al. Hypothermic circulatory arrest with moderate, deep or profound hypothermic selective antegrade cerebral perfusion: which temperature provides best brain protection? Eur J Cardiothorac Surg 2006;30:492-8. [Crossref] [PubMed]
- Numata S, Tsutsumi Y, Monta O, et al. Aortic arch repair with antegrade selective cerebral perfusion using mild to moderate hypothermia of more than 28°C. Ann Thorac Surg 2012;94:90-5; discussion 95-6. [Crossref] [PubMed]
- Qing M, Vazquez-Jimenez JF, Klosterhalfen B, et al. Influence of temperature during cardiopulmonary bypass on leukocyte activation, cytokine balance, and post-operative organ damage. Shock 2001;15:372-7. [Crossref] [PubMed]
- Coselli JS, LeMaire SA. Tips for Successful outcomes for descending thoracic and thoracoabdominal aortic aneurysm procedures. Semin Vasc Surg 2008;21:13-20. [Crossref] [PubMed]
- Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg 1986;3:389-404. [Crossref] [PubMed]
- Schepens M, Dossche K, Morshuis W, et al. Introduction of adjuncts and their influence on changing results in 402 consecutive thoracoabdominal aortic aneurysm repairs. Eur J Cardiothorac Surg 2004;25:701-7. [Crossref] [PubMed]
- Gallinat A, Fox M, Lüer B, et al. Role of pulsatility in hypothermic reconditioning of porcine kidney grafts by machine perfusion after cold storage. Transplantation 2013;96:538-42. [Crossref] [PubMed]
- Sullivan TM, Hertzer NR. Stenting of the Renal Artery to Improve Renal Function Prior to Thoracoabdominal Aneurysm Repair. J Endovasc Surg 1998;5:56-9. [Crossref] [PubMed]
- Yue RL, Collins TJ, Sternbergh WC 3rd, et al. Acute renal failure after redo thoracoabdominal aortic aneurysm repair in a patient with a solitary kidney: successful percutaneous treatment. J Endovasc Ther 2000;7:399-403. [Crossref] [PubMed]
- LeMaire SA, Jamison AL, Carter SA, et al. Deployment of balloon expandable stents during open repair of thoracoabdominal aortic aneurysms: a new strategy for managing renal and mesenteric artery lesions. Eur J Cardiothorac Surg 2004;26:599-607. [Crossref] [PubMed]
- Kalder J, Keschenau P, Hanssen SJ, et al. The impact of selective visceral perfusion on intestinal macrohemodynamics and microhemodynamics in a porcine model of thoracic aortic cross-clamping. J Vasc Surg 2012;56:149-58. [Crossref] [PubMed]
- Kalder J, Ajah D, Keschenau P, et al. Microcirculatory perfusion shift in the gut wall layers induced by extracorporeal circulation. J Vasc Surg 2015;61:497-503. [Crossref] [PubMed]
- Mori Y, Kamada T, Ochiai R. Reduction in the incidence of acute kidney injury after aortic arch surgery with low-dose atrial natriuretic peptide: a randomised controlled trial. Eur J Anaesthesiol 2014;31:381-7. [Crossref] [PubMed]
- Sezai A, Shiono M, Orime Y, et al. Low-dose continuous infusion of human atrial natriuretic peptide during and after cardiac surgery. Ann Thorac Surg 2000;69:732-8. [Crossref] [PubMed]
- Sezai A, Hata M, Niino T, et al. Continuous low-dose infusion of human atrial natriuretic peptide in patients with left ventricular dysfunction undergoing coronary artery bypass grafting. J Am Coll Cardiol 2010;55:1844-51. [Crossref] [PubMed]
- Swärd K, Valsson F, Odencrants P, et al. Recombinant human atrial natriuretic peptide in ischemic acute renal failure: a randomized placebo-controlled trial. Crit Care Med 2004;32:1310-5. [Crossref] [PubMed]
- Gifford E, Kalra M, Pochettino A, et al. Early and late results of reconstruction with renal and visceral bypasses during open thoracoabdominal aortic aneurysm repair. J Vasc Surg 2017;6:142S. [Crossref]
- Lachat M, Mayer D, Criado FJ, et al. New Technique to facilitate renal revascularization with use of telescoping self-expanding stent grafts: VORTEC. Vascular 2008;16:69-72. [Crossref] [PubMed]
- Eide TO, Romundstad P, Saether OD, et al. A strategy for treatment of type III and IV thoracoabdominal aortic aneurysm. Ann Vasc Surg 2004;18:408-13. [Crossref] [PubMed]
- Eldrup-Jorgensen J, Bredenberg CE. Repair of type III and type IV thoracoabdominal aortic aneurysms by using a long beveled anastomosis: A description of technique. Surgery 1998;123:351-5. [Crossref] [PubMed]
- Safi HJ, Estrera AL, Miller CC, et al. Evolution of risk for neurologic deficit after descending and thoracoabdominal aortic repair. Ann Thorac Surg 2005;80:2173-9. [Crossref] [PubMed]
- Svensson LG, Crawford ES, Hess KR, et al. Thoracoabdominal aortic aneurysms associated with celiac, superior mesenteric, and renal artery occlusive disease: methods and analysis of results in 271 patients. J Vasc Surg 1992;16:378-89; discussion 389-90. [Crossref] [PubMed]
- Bornak A, Goldstein LJ, Rey J, et al. Aortic aneurysmal repair with surtureless visceral revascularization using novel hybrid vascular graft and a gradual funneling technique. Vasc Endovascular Surg 2012;46:258-61. [Crossref] [PubMed]
- Panneton JM, Hollier LH. Nondissecting thoracoabdominal aortic aneurysms: Part I. Ann Vasc Surg 1995;9:503-14. [Crossref] [PubMed]
- Piazza M, Ricotta JJ. Open surgical repair of thoracoabdominal aortic aneurysms. Ann Vasc Surg 2012;26:600-5. [Crossref] [PubMed]
- Back MR, Bandyk M, Bradner M, et al. Critical analysis of outcome determinants affecting repair of intact aneurysms involving the visceral aorta. Ann Vasc Surg 2005;19:648-56. [Crossref] [PubMed]
- Dubois L, Durant C, Harrington DM, et al. Technical factors are strongest predictors of postoperative renal dysfunction after open transperitoneal juxtarenal abdominal aortic aneurysm repair. J Vasc Surg 2013;57:648-54. [Crossref] [PubMed]
- Estrera AL, Sandhu HK, Charlton-Ouw KM, et al. A Quarter Century of Organ Protection in Open Thoracoabdominal Repair. Ann Surg 2015;262:660-8. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Semb G, Krog J, Johansen K. Renal metabolism and blood flow during local hypothermia, studied by means of renal perfusion in situ. Acta Chir Scand Suppl 1960;253:196-202. [PubMed]
- Harvey RB. Effects of temperature on function of isolated dog kidney. Am J Physiol 1959;197:181-6. [Crossref] [PubMed]
- Levy MN. Oxygen consumption and blood flow in the hypothermic perfused kidney. Am J Physiol 1959;197:1111-4. [Crossref] [PubMed]
- Allen BT, Anderson CB, Rubin BG, et al. Preservation of renal function in juxtarenal and suprarenal abdominal aortic aneurysm repair. J Vasc Surg 1993;17:948-58; discussion 958-9. [Crossref] [PubMed]
- Black JH. Technique for repair of suprarenal and thoracoabdominal aortic aneurysms. J Vasc Surg 2009;50:936-41. [Crossref] [PubMed]
- Scrascia G, Guida P, Rotunno C, et al. Anti-inflammatory strategies to reduce acute kidney injury in cardiac surgery patients: A meta-analysis of randomized controlled trials. Artif Organs 2014;38:101-12. [Crossref] [PubMed]
- Turner S, Derham C, Orsi NM, et al. Randomized clinical trial of the effects of methylprednisolone on renal function after major vascular surgery. Br J Surg 2008;95:50-6. [Crossref] [PubMed]
- Flores J, DiBona DR, Beck CH, et al. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest 1972;51:118-26. [Crossref] [PubMed]
- Hanssen SJ, Derikx JP, Vermeulen Windsant IC, et al. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann Surg 2008;248:117-25. [Crossref] [PubMed]
- Afnan J, Ahmadi-Yazdi C, Sheu EG, et al. Inhibition of rat gut reperfusion injury with an agent developed for the mouse. Evidence that amplification of injury by innate immunity is conserved between two animal species. Am J Physiol Regul Integr Comp Physiol 2010;298:R1675-81. [Crossref] [PubMed]
- Liu SH, Ma K, Xu XR, et al. A single dose of carbon monoxide intraperitoneal administration protects rat intestine from injury induced by lipopolysaccharide. Cell Stress Chaperones 2010;15:717-27. [Crossref] [PubMed]
- Petrat F, Rönn T, De Groot H. Protection by pyruvate infusion in a rat model of severe intestinal ischemia-reperfusion injury. J Surg Res 2011;167:e93-101. [Crossref] [PubMed]
- Petrat F, De Groot H. Protection against severe intestinal ischemia/reperfusion injury in rats by intravenous resveratrol. J Surg Res 2011;167:e145-55. [Crossref] [PubMed]
- Tsunooka N, Maeyama K, Hamada Y, et al. Bacterial translocation secondary to small intestinal mucosal ischemia during cardiopulmonary bypass. Measurement by diamine oxidase and peptidoglycan. Eur J Cardiothorac Surg 2004;25:275-80. [Crossref] [PubMed]
- van Saene HK, Silvestri L, Taylor N, et al. Treatment of sepsis. Lancet Infect Dis 2012;12:745-6. [Crossref] [PubMed]
- Silvestri L, De La Cal MA, Van Saene HK. Selective decontamination of the digestive tract: The mechanism of action is control of gut overgrowth. Intensive Care Med 2012;38:1738-50. [Crossref] [PubMed]
Cite this article as: Waked K, Schepens M. State-of the-art review on the renal and visceral protection during open thoracoabdominal aortic aneurysm repair. J Vis Surg 2018;4:31.


