Application of the coaxial smart drain in patients with a large air leak following anatomic lung resection: a prospective multicenter phase II analysis of efficacy and safety
Introduction
The presence of air leak following lung resection remains a frequent problem, which may prolong hospital stay and increase hospital costs (1-4). In addition, the incidence of air leak following lung resection remained somewhat unchanged even after the widespread use of minimally invasive thoracic surgery (5,6)
In the past, some studies documented the efficacy of soft and flexible chest tube (e.g., spiral-drains) in patients who underwent thoracic surgery, by reducing the intercostal pain, increasing patient comfort and in correctly monitoring post-operative bleeding and typical air or fluid leak (7-11). Nevertheless, safety in case of post-operative large air or liquid leak remains questionable (10-12) and the quantitative evaluation of massive air drainage has been reported to be difficult (11).
The coaxial chest tube (Coaxial Drain, Redax S.p.A, Poggio Rusco Mantova, Italy—Figure 1) has been designed to overcome this limitation as it combines an external spiral drain to facilitate drainage of pleural effusions and an internal coaxial drain for air drainage (Figure 2).
Although the Coaxial Drain has been regularly used in several thoracic units, it has never been formally tested for its safety in a population of lung resection patients with measured air leak following operation.
The objective of this study was to verify through a multicentre study the safety and the effectiveness of this novel chest tube in a consecutive series of selected patients who underwent anatomical pulmonary resection and with an active and large air leak presence at the end of the procedure, objectively measured using digital chest drainage system.
Methods
Between October 2016 and September 2017, data from patients submitted to lung resection with curative intent and operated by two consultants in two Department of Thoracic Surgery of two different centers [i.e., St. James’s University Hospital (Leeds, UK) and Azienda Ospedaliera Universitaria Città della Salute e della Scienza di Torino (Turin, Italy)] were prospectively collected.
The inclusion criteria consisted in (I) the use of one single 28 Fr coaxial smart drain (II) the presence of an air leak greater than 50 mL/min, measured with a digital drainage system, during the first 6 postoperative hours (III) the performed surgical procedure consisting in an anatomical lung resections (lobectomy, bilobectomy or segmentectomy) for lung cancer. Patients treated with preoperative protocols (i.e., chemotherapy, radiotherapy) were also included.
The absence of postoperative air leak, an extended resection (i.e., combined lung and chest wall/diaphragm resections), pneumonectomy or a non-anatomical sublobar resection (e.g., wedge resection) represented the exclusion criteria from this study.
Surgical procedures were performed either through video-assisted thoracic surgery (VATS) (Figure 3) or muscle sparing thoracotomy. At the end of each surgical procedure, the coaxial smart drain was placed on −20 cmH2O suction; air and fluid leak were monitored with using a digital drainage system (Drentech™ Palm Evo, Redax S.p.A, Poggio Rusco Mantova, Italy or Thopaz, Medela Healthcare, Baar, Switzerland). A chest X-ray (CXR) was performed in the postoperative day (POD) 1. The chest drain algorithm for its removal was in accordance with institutional protocols as previously described (13,14).
Written informed consent was obtained in all patients.
Statistical analysis
Categorical data are presented as a number (percentage, %), and continuous data as the median with IQR.
A descriptive statistics was used to report the incidence of complications assumed to be associated with the use of the coaxial drain. The analysis was performed on Stata 12.0 statistical software (Stata Co. College Station, TX, USA).
Results
According to the selection criteria, 48 consecutive patients (33 in Leeds Center and 15 in Torino Center) (27 males) submitted to lobectomy (37 patients, 77%) or anatomic segmentectomies (11 patients) were included in the analyses. Thirty-four operations (71%) were performed by VATS with 3 conversions due to oncologic reasons. These patients represent 38% of all patients submitted to anatomic lung resections for cancer and operated on during the same period by the same surgeons in the two hospitals.
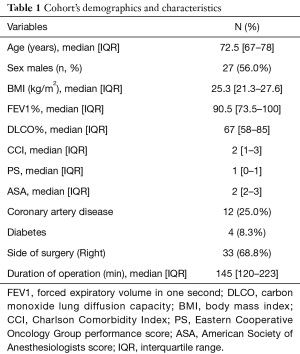
Patients’ demographics and clinical, radiological, surgical and pathologic characteristics of these patients are reported in Table 1. Most of the patients were male (27–56%), and the median age at the time of surgery was 72.5 years (IQR, 67–78).
Full table
The most frequently performed operation was right upper lobectomy (18 cases, 38%). Right lower lobectomy was performed in 8 cases (17%); left upper and left lower lobectomies were performed in 6 and 5 cases, respectively.
Outcome
The median duration of chest tubes was 13 days (IQR, 4–19) and the longest duration of the coaxial drain was 35 days. The median duration of air leak was 9 days (IQR, 2–17.5). The longest air leak duration documented was 35 days. The median postoperative hospital stay was 7.5 days (IQR, 5–10.5). Twelve (25%) patients had a partially unexpanded lung at the first chest X ray obtained but none of them required additional procedure or clinical intervention. No patient had undrained postoperative pleural effusion judged to require an additional chest tube placement.
Twenty-three patients (48%) were discharged from the hospital with the chest tube in place. There were no problems reported by the patients in the management of the chest tube at home or any drainage-related complication.
Ten (21%) patients had partially collapsed lung after chest drain removal, but only 4 were symptomatic and required another drain placement. One patient required re-operation for empyema. There were 12 (25%) cases of clinically or radiologically significant surgical emphysema; in none of these patients any additional procedure or re-operation were required, and they were treated conservatively by increasing the level of suction. Three (6%) patients in this series developed empyema (one requiring surgery), 3 had pneumonia, 1 developed atrial fibrillation, 1 atelectasis requiring bronchoscopy and 1 acute renal failure. Two patients died because of pneumonia and respiratory failure.
Discussion
Managing of chest drainage in thoracic surgery is still founded on the clinician individual experience and belief, more than on well-established scientific evidence (3,15,16). Nevertheless, chest drain is one of the most important determinant of patient’s pain and morbidity. In addition, chest drain management influences the patients discharge, the overall length of stay and the hospitalization cost (2,3).
The results of our study, conducted in a cohort of patients with an active and large air leak after lung resection detected with digital drainage systems, suggest that the use of this novel coaxial drain was satisfactory with (I) no clinically relevant complication caused by the use of this drain, (II) no need to insert additional drain or replace the existing one with another drain in this series and (III) a duration of air leak and chest tubes as well as the incidence of subcutaneous emphysema was in line with what observed in the daily practice in similar highly selected patients.
The Coaxial Smart Drain is a flexible silastic drain characterized by a round corrugated profile combined with an internal coaxial lumen. This combination, on one hand, allows the independent evacuation of fluid (Figure 4) and air (Figure 5) that could promote a more effective drainage; on the other hand, prevents the occlusion of the tube due to kinking or twisting. Indeed, traditional stiff chest tubes, due to their intrinsic rigidity, could ease the continuation of air leaks hindering the correct contact between lung and parietal pleura, this by acting as a physical impediment or by the heterogeneous aspiration through the drain holes (10,17). Furthermore, it is well known that due to their size and rigidity, stiff drains may obstacle early mobilization, deep breathing and aggravate pain at removal. As matter of fact, the grooved surface of the silastic drain allows a constant suction (by capillary action), over the whole corrugated portion of the tube (18-20). Moreover, flexible drains are described as more comfortable for the patient and less painful at their removal (7,9,10).
In recent years, the use of other types of silastic drains after both cardiac (21,22), and thoracic surgery (8-11) have been reported in some mono-institutional experiences; commonly, these studies reported the results of the use of small-bore drainages (usually 19-french), without coaxial lumen and without a digital monitoring of the air leak. Undeniably, the use of such flexible drains showed to positively influence patient pain and comfort (7,9,10,21,22) and to reduce hospitalization (7,8,10). However, some authors reported a not negligible number of complication (e.g., tube dislodgement, persistent air leakage, tension pneumothorax, not-recognized bleeding) requiring additional procedures such as additional chest drain insertion. (9,12). One possible reason might be the association of the small size and flexibility of these drains that could predispose to dislodgement, twisting and torsion and clogging of the drain; the latter also favored by the hypercoagulability after lung surgery (11). Moreover, Sakakura et al. showed that the air evacuation performance of a 24- and 19-Frech silastic drain is similar to that of a 16- and 12-Frech traditional stiff chest tubes, respectively (11).
Therefore, effectiveness and safety of the use of silastic chest drains in patients with a high risk of active, large and prolonged air leak is still matter of debate (10).
The Coaxial Smart Drain has been designed to overcome the limitations of small silastic drains; maintaining at the same time the advantages given by the flexibility and by the capillarity effect of the grooved profile.
In our cohort of patients with large air leak after lung resections, objectively assessed by a digital drainage system, we did not experience any complication due to the drain such as dislodgment, occlusion, pneumothorax or unrecognized bleeding requiring an additional chest tube insertion. This was presumably related to the addition of the non-collapsible internal coaxial lumen within the corrugated profile allowing an optimal fluid and air evacuation and preventing kinking and occlusion of the tube (18).
The main limitation of this study is the absence of a control group with use of a traditional chest drain. However, due to the design of the study (inclusion of patients with large air leak assessed during the first 6 hours after the operation) and the relatively small number of events (patients with large air leak at tend of the procedure) a randomized trial could be difficult to implement. Nevertheless, this study represents a not-underestimate and actual snapshot inferable from real clinical practice (23).
In conclusion, our experience with this novel Coaxial Drain was satisfactory with no clinically relevant complication attributable to its use. Moreover, additional drainage insertion or Coaxial Drain replacement were not required. The duration of air leak and chest tubes, as well as the incidence of subcutaneous emphysema, appears to be in line with what observed in the daily practice in this selected population of patients with active and large air leak after surgery. Based on these results we started to systematically use this novel type of chest tube in all patients undergoing lung resections.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was regarded as service evaluation by the R & I Department of the Hospital not requiring ethical committee approval. Written informed consent was obtained in all patients.
References
- Cerfolio RJ, Varela G, Brunelli A. Digital and smart chest drainage systems to monitor air leaks: the birth of a new era? Thorac Surg Clin 2010;20:413-20. [Crossref] [PubMed]
- Filosso PL, Sandri A, Guerrera F, et al. Management of Chest Drains After Thoracic Resections. Thorac Surg Clin 2017;27:7-11. [Crossref] [PubMed]
- Filosso PL. Chest Drainage Management: Where Are We Now? Thorac Surg Clin 2017;27:ix. [Crossref] [PubMed]
- Varela G, Jiménez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Ishikura H, Kimura S. The use of flexible silastic drains after chest surgery: novel thoracic drainage. Ann Thorac Surg 2006;81:331-3. [Crossref] [PubMed]
- Icard P, Chautard J, Zhang X, et al. A single 24F Blake drain after wedge resection or lobectomy: a study on 100 consecutive cases. Eur J Cardiothorac Surg 2006;30:649-51. [Crossref] [PubMed]
- Kejriwal NK, Newman MA. Use of a single silastic chest drain following thoracotomy: initial evaluation. ANZ J Surg 2005;75:710-2. [Crossref] [PubMed]
- Terzi A, Feil B, Bonadiman C, et al. The use of flexible spiral drains after non-cardiac thoracic surgery. A clinical study. Eur J Cardiothorac Surg 2005;27:134-7. [Crossref] [PubMed]
- Sakakura N, Fukui T, Mori S, et al. Fluid drainage and air evacuation characteristics of Blake and conventional drains used after pulmonary resection. Ann Thorac Surg 2009;87:1539-45. [Crossref] [PubMed]
- Clark G, Licker M, Bertin D, et al. Small size new silastic drains: life-threatening hypovolemic shock after thoracic surgery associated with a non-functioning chest tube. Eur J Cardiothorac Surg 2007;31:566-8. [Crossref] [PubMed]
- Filosso PL, Nigra VA, Lanza G, et al. Digital versus traditional air leak evaluation after elective pulmonary resection: a prospective and comparative mono-institutional study. J Thorac Dis 2015;7:1719-24. [PubMed]
- Brunelli A, Thomas C, Dinesh P, et al. Enhanced recovery pathway versus standard care in patients undergoing video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2017;154:2084-90. [PubMed]
- Novoa NM, Jiménez MF, Varela G. When to Remove a Chest Tube. Thorac Surg Clin 2017;27:41-6. [Crossref] [PubMed]
- Varela G, Jiménez MF, Novoa NM, et al. Postoperative chest tube management: measuring air leak using an electronic device decreases variability in the clinical practice. Eur J Cardiothorac Surg 2009;35:28-31. [Crossref] [PubMed]
- Stahly TL, Tench WD. Lung entrapment and infarction by chest tube suction. Radiology 1977;122:307-9. [Crossref] [PubMed]
- Rena O, Parini S, Papalia E, et al. The Redax(®) Coaxial Drain in pulmonary lobectomy: a study of efficacy. J Thorac Dis 2017;9:3215-21. [Crossref] [PubMed]
- Stolz AJ, Lischke R, Simonek J, et al. (Comparison study on the use of tubular and spiral thoracic drains following lung resections. A prospective study). Rozhl Chir 2005;84:529-32. [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. The use of Blake drains following general thoracic surgery: is it an acceptable option? Interact Cardiovasc Thorac Surg 2009;8:58-61. [Crossref] [PubMed]
- Lancey RA, Gaca C, Vander Salm TJ. The use of smaller, more flexible chest drains following open heart surgery: an initial evaluation. Chest 2001;119:19-24. [Crossref] [PubMed]
- Obney JA, Barnes MJ, Lisagor PG, et al. A method for mediastinal drainage after cardiac procedures using small silastic drains. Ann Thorac Surg 2000;70:1109-10. [Crossref] [PubMed]
- Guerrera F, Renaud S, Tabbò F, et al. How to design a randomized clinical trial: tips and tricks for conduct a successful study in thoracic disease domain. J Thorac Dis 2017;9:2692-6. [Crossref] [PubMed]
Cite this article as: Guerrera F, Filosso PL, Pompili C, Olivetti S, Roffinella M, Imperatori A, Brunelli A. Application of the coaxial smart drain in patients with a large air leak following anatomic lung resection: a prospective multicenter phase II analysis of efficacy and safety. J Vis Surg 2018;4:26.