Thoracic endovascular aortic repair (TEVAR) versus open versus medical management of type B dissection
Despite improvements in diagnosis and management, aortic dissection remains a lethal disease. Type B aortic dissection (TBAD) comprises approximately one third of all acute aortic dissections with management of this disease undergoing revolutionary changes since the introduction of thoracic endovascular aortic repair (TEVAR) (1). Acute dissections are defined as those with delay from onset of symptoms to presentation shorter than 14 days. Approximately 25–40% of TBAD are considered complicated, with at least one of the following characteristics: end organ or lower extremity malperfusion, rupture, shock, neurologic compromise, refractory pain, refractory hypertension, or early progression of disease (2).
In the past, complicated TBADs were mostly patients presenting with malperfusion or rupture. A more recent review of the International Registry of Acute Aortic Dissection (IRAD) demonstrated that patients with refractory hypertension had a greater than 20-fold increase in mortality when managed with medical therapy alone. Refractory pain was also identified as a predictor of dismal outcome with isolated medical management having an in-hospital mortality rate of 35.6% (3). Detailed analysis of large cohorts of TBAD patients have helped refine the definition of complicated to better identify those patients with high risk features that may benefit from interventions such as TEVAR or open surgical repair.
Although the literature on TBAD is heterogeneous with respect to management and outcomes, consensus is building on favorable treatment algorithms (1,4). Widely agreed upon is the improvement in early outcomes seen with TEVAR for acute complicated TBAD. Although there have been no randomized controlled trials comparing the three strategies for acute, complicated TBAD (medical therapy, TEVAR, open surgery), TEVAR is now considered first-line therapy for those patients with suitable anatomy. In non-randomized studies, morbidity and mortality were improved with early adoption of endovascular repair (2,5-7).
Medical therapy for acute complicated TBAD remains relevant in that it should be initiated in all patients as soon as a diagnosis of dissection is made. Optimal medical therapy (OMT) consists of anti-impulse therapy with goal systolic blood pressure of 100–120 mmHg and heart rate under 60 beats per minute (2,4-6). Permissive hypotension is appropriate in select patients with hemorrhagic shock or contained rupture to avoid dissection progression or free rupture. Treatment of complicated TBAD with medical therapy alone is most often reported as occurring in patients unsuitable for TEVAR from an anatomic standpoint, or prohibitive risk for an open surgical approach. Mortality at 30 days with optimal medical management in a contemporary series looking only at acute complicated TBAD was 33% (8).
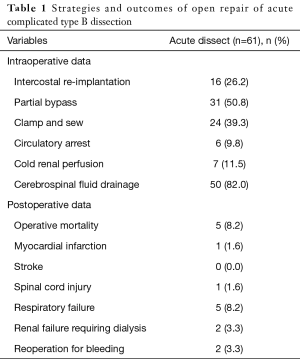
Historical data from the IRAD database reported an in-hospital mortality greater than 30% for patients treated with open surgery (1-3). The contemporary IRAD review showed that open surgical operative mortality has improved in more recent years, possibly due to improvement in patient selection and referrals to high-volume centers of excellence (1). An analysis of our data on open surgical management of acute complicated TBAD supports the concept that open surgery still has a significant role for this high-risk cohort. From 1997 to 2016 we performed open repair on 61 patients presenting with complicated acute TBAD, which we defined as those with evidence of malperfusion, rupture, refractory pain and/or hypertension, and anatomy unsuitable for TEVAR. Operative mortality was 8.2% with only one patient experiencing a major neurologic complication. Major adverse events including stroke, spinal cord injury, myocardial infarction and dialysis dependent renal failure were limited. Table 1 outlines the intraoperative strategies and postoperative outcomes. While TEVAR remains the preferred strategy for acute complicated TBAD, some patients will not meet anatomic criteria for endovascular repair. In addition, patients with connective tissue disorders may not be ideal candidates. TEVAR may stabilize those with life-threatening conditions. However, careful follow-up is mandatory to identify those with disease progression or significant complications that may require open repair for resolution of their disease. Our data suggest that competitive outcomes are possible in high-volume aortic centers for patients unsuitable for endovascular therapy.
Full table
After its introduction in the 1990s, the results for TEVAR reflected a vast improvement in mortality compared to historical data on open surgical repair and OMT. The Valiant United States IDE Study was a pivotal prospective trial examining 50 patients who were treated with TEVAR for acute complicated TBAD. Thirty-day and 12-month mortality were 8% and 15%, respectively. Spinal ischemia was 6%, and overall 30-day serious adverse events occurred in 38% of patients, which was comparable to other studies and led to FDA approval of the device (7). Mid- and long-term data are becoming available, with one recent single-center retrospective review of 50 patients achieving an overall survival at 5 years of 84%, with 26% of patients requiring reintervention for either branch vessel compromise or endoleaks (9). Fortunately for those requiring reintervention, 65% could be performed utilizing isolated endovascular or hybrid repair techniques.
Famularo et al., recently examined studies following aortic dimensions after TEVAR for TBAD to determine the incidence of aneurysmal degeneration (10). Eleven studies were identified containing data on acute dissections, all of which included but were not limited to complicated acute TBAD. In their analysis of the acute aortic dissection patients, the incidence of significant thoracic aortic growth ranged widely from 8% to 63%. Growth was seen even in those with documented aortic remodeling. They concluded that caution should be exercised when interpreting a decrease in false lumen diameter as a marker indicative of limited late aortic expansion. This information, in combination with the high incidence of reintervention, highlights the mandate for continued surveillance of all patients after TEVAR for acute TBAD.
While there is no doubt that the evolution of TEVAR changed the landscape of therapy and prognosis for patients with acute complicated TBAD, it is not currently suitable for every patient and has a substantial need for early and late reintervention. OMT should not stand alone as treatment for these patients, but should be instituted as soon as the diagnosis is made and continued around the time of any intervention, endovascular or open. OMT similarly continues to be important after repair to delay progression of a disease process that has shown itself to be dynamic and ongoing. TEVAR is the first-line treatment for acute complicated TBAD and should be pursued once the diagnosis is made. Additional follow-up is needed to manage expectations for long-term outcomes before expanding the indications for TEVAR even further. In stable patients who are not suitable candidates for endovascular therapy, transfer to a high-volume aortic center should be considered a viable option as results in this setting may rival that seen with less invasive options. Ongoing improvements in both endovascular and open repair of the thoracic aorta may further refine treatment algorithms as we analyze both the short- and long-term strategies and outcomes for these critically ill patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Alfson DB, Ham SW, Type B. Aortic Dissections: Current Guidelines for Treatment. Cardiol Clin 2017;35:387-410. [Crossref] [PubMed]
- Patel AY, Eagle KA, Vaishnava P. Acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection. Ann Cardiothorac Surg 2014;3:368-74. [PubMed]
- Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. Erratum in: Circulation 2010;122:e410. [Crossref] [PubMed]
- Bavaria JE, Brinkman WT, Hughes GC, et al. Outcomes of Thoracic Endovascular Aortic Repair in Acute Type B Aortic Dissection: Results From the Valiant United States Investigational Device Exemption Study. Ann Thorac Surg 2015;100:802-8; discussion 808-9. [Crossref] [PubMed]
- Zeeshan A, Woo EY, Bavaria JE, et al. Thoracic endovascular aortic repair for acute complicated type B aortic dissection: superiority relative to conventional open surgical and medical therapy. J Thorac Cardiovasc Surg 2010;140:S109-15; discussion S142-6.
- Hanna JM, Andersen ND, Ganapathi AM, et al. Five-year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg 2014;59:96-106. [Crossref] [PubMed]
- Famularo M, Meyermann K, Lombardi JV. Aneurysmal degeneration of type B aortic dissections after thoracic endovascular aortic repair: A systematic review. J Vasc Surg 2017;66:924-30. [Crossref] [PubMed]
Cite this article as: Iannacone E, Girardi L. Thoracic endovascular aortic repair (TEVAR) versus open versus medical management of type B dissection. J Vis Surg 2018;4:8.


