Thoracoscopic lobectomy after induction therapy—a paradigm shift?
Introduction
Over the last two decades, significant advances have been made in the management of patients with early stage non-small cell lung cancer (NSCLC). Importantly, video-assisted thoracoscopic surgery (VATS) has emerged as an acceptable alternative, and in many cases the preferred alternative, to open approaches (thoracotomy), with comparable long-term outcomes and overall oncologic efficacy (1,2). In addition, the experiences gained from thoracoscopic approaches—together with concurrent improvements in instrumentation and video technology—have enabled the evolution thoracoscopic lobectomy in the management of patients with locally advanced NSCLC, including stage IIIA (N2) NSCLC (3-5).
Despite the growing experience in thoracoscopic approaches, there remains substantial variability in widespread adoption of VATS lobectomy following induction therapy. Moreover, considerable controversy exists in this area, particularly around the concept of mediastinal staging of clinically N0 patients (c-N0) and the restaging after induction therapy. Current National Comprehensive Cancer Network (NCCN) guidelines for instance, recommend invasive mediastinal staging before surgical resection for most patients with clinical stage I (c-stage I) or II lung cancer, while preoperative invasive mediastinal staging may be appropriate for a strong clinical suspicion of N2 or N3 nodal disease, or when intraoperative cytology or frozen section analysis is not available (6,7).
In addition, there is not a consensus what features constitute “operable” stage IIIA NSCLC: single versus multi-station disease; microscopic involvement only versus bulky disease; and the role of pneumonectomy after induction therapy. Finally, the utility of induction chemotherapy versus chemoradiation, and the subsequent utilization of VATS or thoracotomy are still debated. The resolution of these controversies has been by impaired by biases in established practices, perceived technical difficulty of the resection following induction therapy, and in some cases, limited experience with thoracoscopic strategies in this unique cohort.
In this perspective, we will examine the importance of surgical staging and restaging of the mediastinum for patients with potentially resectable N2 NSCLC, review contemporary data to determine the optimal induction treatment strategy for N2 disease, and finally explore the role of thoracoscopic lobectomy in the management of patients with stage IIIA (N2) NSCLC following induction therapy.
Role of mediastinal staging and re-staging
Accurate assessment of nodal status (mediastinal staging) in lung cancer is crucial for planning therapy and assessing prognosis (8). Despite improvements in advanced imaging modalities such as computed tomography (CT) and positron emission tomography (PET) scans, surgical staging remains the gold standard approach for accurate staging of the mediastinum. Even though PET scans can improve detection of distant metastasis in patients with NSCLC, they provide limited information in terms of confirming or excluding mediastinal tumor involvement. In-fact, multiple studies have shown that mediastinal staging with PET scans for c-stage I patients has a reported sensitivity of 61% to 82%, and specificity of 77% to 82% (9,10). This means that almost 25% of c-stage I patients are incorrectly staged without endobronchial ultrasound (EBUS) or mediastinoscopy. Thus, current NCCN guidelines recommend pathologic mediastinal lymph node evaluation during the pre-treatment evaluation of patients with stage 1B (peripheral T2a, N0), stage 1 (central T1ab to T2a, N0), stage II (T1ab to 2ab, N1 or T2b, N0), stage IIB (T3, N0), and stage IIIA (T3, N1) disease, while consideration in patients with stage IA disease (peripheral T1ab, N0) (6,7).
Likewise, understanding the utility of mediastinal staging and re-staging in the context of potentially resectable N2 NSCLC is also extremely crucial. Re-staging allows for objective assessment of tumor response to treatment (induction therapy), and helps guide further treatment. Thus, the strategy of induction therapy and re-staging remains the standard of care. Induction chemotherapy tests the biology of the tumor, and those patients that do not response, they tend to have poor prognosis. Importantly however, if radiation therapy is also used, the strategy of re-staging to determine operability becomes meaningless. While some clinicians may think restaging is not important, survival advantage of down-staged patients suggest that re-staging is useful. For instance, Betticher et al. examined 90 patients with IIIA (pN2) after 3 cycles of chemotherapy (docetaxel/cisplatin) and resection, and found that mediastinal down-staging and complete resection was associated with improved survival (11).
In terms of diagnostic modalities, re-staging after induction therapy should include PET for distant metastatic assessment, but neither PET nor EBUS although are effective in staging the mediastinum after induction therapy (7). Instead, mediastinoscopy (or repeat mediastinoscopy) or VATS is recommended, if pathologic assessment of the mediastinum would be used to assign therapy. Based on our experience, staging and re-staging can be grouped into 2 dominant strategies including EBUS prior to induction and restaging with video-mediastinoscopy, or mediastinoscopy prior to induction and restaging with VATS or repeat mediastinoscopy (Table 1). Notably, the feasibility and safety of repeat mediastinoscopy in restaging of lung cancer after induction therapy has been demonstrated by multiple studies. For instance, Lardinois and his colleagues compared 219 patients who underwent video mediastinoscopy without induction therapy to 24 patients who underwent video mediastinoscopy following induction therapy, and found that video mediastinoscopy after induction therapy for NSCLC was as accurate and did not confer additional morbidity (12). Moreover, sensitivity and specificity of video mediastinoscopy with versus without induction therapy was also comparable (81% vs. 87%, and 91% vs. 96%, respectively) (12). Similarly, Call et al. examined their experience in 96 patients who underwent re-mediastinoscopy (84 were re-staged after N2 disease), and found sensitivity of 74%, accuracy of 87% but interestingly a median survival of 51.5 months for true node-negative patients and versus 11 months for false node-negative (i.e., node positive) (13).
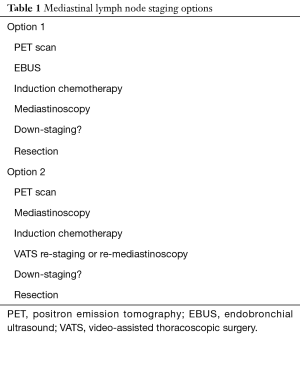
Full table
Even though re-mediastinoscopy after induction therapy is feasible, it is significantly more difficult than primary mediastinoscopy, is often associated with higher complication and false negative rates, and not adequate especially for lymph node stations 5, 6, 8 and 9. More recently, VATS mediastinal lymph node restaging has been introduced to improve the efficacy of mediastinal assessment after induction therapy (14,15). VATS staging and restaging allows for evaluation of lymph node stations 2R, 4R, 5, 6, 7, 8, 9, 10, 11, 12 and pleura, allows for easier acquisition of larger biopsies, and also facilitates the intended resection (i.e., lobectomy). VAT re-staging also allows for resection all ipsilateral nodes (not just a biopsy) to accurately access tumor response. According to one prospective, multi-institutional study for instance, VATS restaging after induction therapy for NSCLC has a sensitivity of 75%, specificity of 100%, and accuracy of 76% (16).
Existing data on induction therapy for N2 disease
Patients with N2 disease can be challenging to manage as they often have varying levels of lymph node involvement (8). They can be treated with induction chemotherapy, chemoradiotherapy followed by surgery, or definitive chemoradiotherapy without surgery (7). Existing guidelines for T1-2, T2 (other than invasive), N2 nodes positive, M0 patients recommend either definitive concurrent chemoradiation or induction chemotherapy with or without radiation therapy. Furthermore, patients with local disease progression are given radiation therapy (if not given) with chemotherapy, while those without progression may be amenable to surgery, depending on tumor response (7,17).
Nonetheless, use of induction chemotherapy versus chemoradiation is not only of immense interest but is also highly debated, in part due to the limited number of randomized studies to help guide evidence-based decision making. Interestingly, recent studies have shown no additional benefit of radiation therapy to induction chemotherapy. For instance, Pless et al. (18) randomly assigned 232 stage IIIA (N2) patients from 23 centers in Switzerland, Germany and Serbia to receive either to neoadjuvant chemoradiation (3 cycles cisplatin, docetaxel followed by radiotherapy) or chemotherapy alone. The study found that both groups had similar median event-free survival and overall survival, radiotherapy did not add any benefit to induction chemotherapy followed by surgery. Similarly, Katakami et al. (19) randomized 60 patients to either chemoradiation or chemotherapy alone followed by surgery, and found no difference in progression-free survival and overall survival between the groups, although addition of radiation therapy conferred better local control without significant adverse effects. This study, however, was terminated prematurely due to slow accrual.
Our institution further examined outcomes between induction chemotherapy and induction chemoradiation using the National Cancer Data Base, and found that Induction chemoradiation was not associated with a survival benefit compared with induction chemotherapy, although down staging from N2 to N0/N1 was more common with induction chemoradiation compared with induction chemotherapy (58% vs. 46%, P<0.01) (20). Likewise, a systematic review and meta-analysis by Shah et al. (21), demonstrated no survival benefit to adding radiation to induction chemotherapy versus induction chemotherapy alone, although the addition of radiation was associated with a higher complication rate.
Role of thoracoscopic lobectomy
There is significant variation in the use of thoracoscopic lobectomy among various institutions, including the treatment of both early stage NCSLC and locally advanced NSCLC. Furthermore, as discussed above, there is a lack of consensus on the management of Stage IIIA NSCLC, highlighting the pivotal role of a multi-disciplinary team to help guide individualized treatment decisions. Per NCCN guidelines, surgery is indicated in stage IIIA with involvement of a single N2 lymph node smaller than 3cm following induction chemotherapy (6,7). There is, however, no consensus on multi-station or bulky disease.
The utility of surgery following induction therapy has been previously demonstrated by multiple studies (3,22,23). For instance, in a phase III randomized trial, Albain and his colleagues have demonstrated that chemotherapy plus radiotherapy with or without resection (preferably lobectomy) are reasonable options for patients with stage IIIA(N2) NSCLC. Likewise, our institution also demonstrated that major lung resection after induction chemotherapy can be performed with acceptable short- and long-term results in appropriately selected patients (22).
More recently, VATS lobectomy has emerged as a safe and effective option following induction therapy, with numerous outcomes advantages and oncologic outcomes at least equivalent to open lobectomy. Kamel and colleagues reported their experience with VATS lobectomy after induction chemotherapy and found no differences in the number of lymph nodes selected, number of stations sampled, and in rate of R0 resection between VATS lobectomy and thoracotomy. Moreover, there was no difference in 5-year disease-free survival in patients who presented with cN2 disease, and VATS was associated with shorter length of stay and a trend towards fewer postoperative complications (24).
Our institutional experience
We examined our own institution long-term outcomes following open versus VATS lobectomy after induction therapy. In our study of 272 lobectomies (25% were VATS while 75% were thoracotomy) after induction chemotherapy, VATS lobectomy had improved 3-year survival compared to thoracotomy, and a trend towards improved long-term survival although not statistically significant. Moreover, there were no differences in rates of postoperative bleeding, atrial fibrillation, respiratory failure or pneumonia (3).
Given our experience, we first proceed with mediastinal staging with mediastinoscopy or EBUS. If single station N2 or non-bulky multi-station N2 is found, we proceed with induction chemotherapy. After completion of induction therapy, we re-stage the patient with PET scans plus either mediastinoscopy (if originally staged with EBUS) or VATS. If there is evidence of down-staging (either radiographically or pathologically), we proceed with resection. If there is minimal down-staging however but with no evidence of progression, we proceed with VATS lobectomy, reserving pneumonectomy for selected patients only.
Conclusions
Management of patients with advanced stage NSCLC continues to evolve with improvements in neoadjuvant treatment therapies, imaging modalities and surgical techniques. Accurate surgical staging and re-staging for patients with potentially resectable stage IIIA (N2) NSCLC is extremely critical in order to help guide clinical decisions. Furthermore, re-staging and demonstration of down-staging is also powerful prognostic factor. The utility of induction radiation in addition to chemotherapy in the contemporary era is also debatable, especially in light of recent evidence. And finally, optimal management after induction therapy involves lobectomy, which can be safely performed via the VATS approach with acceptable survival and oncologic outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yang CJ, Kumar A, Klapper JA, et al. A national analysis of long-term survival following thoracoscopic versus open lobectomy for stage I non-small-cell lung cancer. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Yang CF, D'Amico TA. Open, thoracoscopic and robotic segmentectomy for lung cancer. Ann Cardiothorac Surg 2014;3:142-52. [PubMed]
- Yang CF, Meyerhoff RR, Mayne NR, et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1615-23. [Crossref] [PubMed]
- Yang CF, Kumar A, Gulack BC, et al. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J Thorac Cardiovasc Surg 2016;151:1380-8. [Crossref] [PubMed]
- Yang CJ, Mayne NR, Wang H, et al. Outcomes of major lung resection after induction therapy for non-small cell lung cancer in elderly patients. Ann Thorac Surg 2016;102:962-70. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, Version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: non-small cell lung cancer, Version 4.2016. J Natl Compr Canc Netw 2016;14:255-64. [Crossref] [PubMed]
- Martins RG, D'Amico TA, Loo BW Jr, et al. The management of patients with stage IIIA non-small cell lung cancer with N2 mediastinal node involvement. J Natl Compr Canc Netw 2012;10:599-613. [Crossref] [PubMed]
- Kernstine KH, McLaughlin KA, Menda Y, et al. Can FDG-PET reduce the need for mediastinoscopy in potentially resectable nonsmall cell lung cancer? Ann Thorac Surg 2002;73:394-401; discussion 401-2. [Crossref] [PubMed]
- Gonzalez-Stawinski GV, Lemaire A, Merchant F, et al. A comparative analysis of positron emission tomography and mediastinoscopy in staging non-small cell lung cancer. J Thorac Cardiovasc Surg 2003;126:1900-5. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752-9. [Crossref] [PubMed]
- Lardinois D, Schallberger A, Betticher D, et al. Postinduction video-mediastinoscopy is as accurate and safe as video-mediastinoscopy in patients without pretreatment for potentially operable non-small cell lung cancer. Ann Thorac Surg 2003;75:1102-6. [Crossref] [PubMed]
- Call S, Rami-Porta R, Obiols C, et al. Repeat mediastinoscopy in all its indications: experience with 96 patients and 101 procedures. Eur J Cardiothorac Surg 2011;39:1022-7. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. [Crossref] [PubMed]
- Zhao ZR, Situ DR, Lau RWH, et al. Comparison of Segmentectomy and Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol 2017;12:890-6. [Crossref] [PubMed]
- Jaklitsch MT, Gu L, Demmy T, et al. Prospective phase II trial of preresection thoracoscopic mediastinal restaging after neoadjuvant therapy for IIIA (N2) non-small cell lung cancer: results of CALGB Protocol 39803. J Thorac Cardiovasc Surg 2013;146:9-16. [Crossref] [PubMed]
- Ettinger DS, Riely GJ, Akerley W, et al. Thymomas and thymic carcinomas: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2013;11:562-76. [Crossref] [PubMed]
- Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. [Crossref] [PubMed]
- Katakami N, Tada H, Mitsudomi T, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012;118:6126-35. [Crossref] [PubMed]
- Yang CF, Gulack BC, Gu L, et al. Adding radiation to induction chemotherapy does not improve survival of patients with operable clinical N2 non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:1484-92; discussion 1492-3. [Crossref] [PubMed]
- Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 2012;93:1807-12. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Yang CF, Adil SM, Anderson KL, et al. Impact of patient selection and treatment strategies on outcomes after lobectomy for biopsy-proven stage IIIA pN2 non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1607-13. [Crossref] [PubMed]
- Kamel MK, Nasar A, Stiles BM, et al. Video-assisted thoracoscopic lobectomy is the preferred approach following induction chemotherapy. J Laparoendosc Adv Surg Tech A 2017;27:495-500. [Crossref] [PubMed]
Cite this article as: Hirji SA, Osho A, Balderson SS, D’Amico TA. Thoracoscopic lobectomy after induction therapy—a paradigm shift? J Vis Surg 2017;3:189.


