Robotic-assisted spleen preserving distal pancreatectomy: a technical review
Introduction
Resection of the pancreatic body at any point left of the portal vein is termed distal pancreatectomy (DP) and is traditionally combined with splenectomy. DP with splenic preservation has gained popularity for resection of benign or low-grade malignant lesions of the distal pancreas due to reduced length of postoperative hospital stay and decreased infectious and other severe complications (1,2). Typical indications include: chronic pancreatitis, mucinous cystic neoplasm (MCN), low-grade pancreatic neuroendocrine tumor (PNET), intraductal papillary mucinous neoplasm (IPMN), solid pseudopapillary neoplasm (SPN), and nesidioblastosis (3).
DP is one of the most commonly performed laparoscopic pancreatic surgeries, even considered to be “standard of care” by some authors (4). While laparoscopic DPs still make up the majority of reports in the literature, publication of robotic-assisted DP (RADP) series have steadily increased, confirming the safety and feasibility of the robotic approach (5-10). The da Vinci robotic system provides technical advantages over standard laparoscopy such as stable three-dimensional views, multi-articulated end effectors with seven degrees of freedom, and tremor elimination (11).
While no randomized controlled trial comparing outcomes between laparoscopic DP and RADP has been reported, one retrospective study has suggested that RADP is associated with an increased splenic preservation rate of 95% vs. 28% for laparoscopic DP (9). Other perioperative outcomes have been reported by Zureikat et al. and show that RADP is associated with comparable or even shorter operative times and conversion to laparotomy (6) and no significant differences in postoperative length of stay, pancreatic fistula, blood transfusion, or readmission rate. RADP is also associated with a higher margin negative resection rate and improved lymph node yield versus laparoscopic DP (8). While the procedural cost is higher with the robotic approach, some argue that this is balanced by shorter overall length of stay making RAPD a cost-effective option (11).
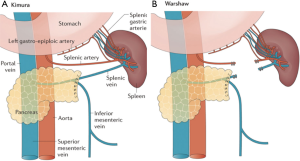
There are two approaches to spleen-preserving DP: vessel-preserving and vessel-sacrificing (12) (Figure 1). Splenic vessel-preservation (Kimura procedure) (13) allows perfusion of the spleen with its native vasculature. This approach can be technically challenging given it requires dissecting the pancreas from the splenic vein which may be closely adherent. Also, there is some controversy regarding the long-term patency of the splenic vein (1,2). Alternatively, the vessel-sacrificing approach (Warshaw procedure) (14), involves segmental resection of both the splenic artery and vein while the gastroepiploic arcade and short gastric arteries are preserved to provide blood supply and drainage of the spleen. This approach may be associated with splenic infarction, abscess or perigastric varices (15,16). A notable limitation of the Warshaw technique is in patients with splenomegaly where blood flow through the short gastric arteries may not support adequate perfusion.
Spleen-preserving RADP remains a procedure primarily performed in a few large-volume centers. In this technical review, we provide a comprehensive description of our approach.
Patient selection and workup
There are no specific contraindications to spleen-preserving RADP other than confirmed or suspected primary pancreatic malignancy since this requires concurrent splenectomy for lymph node sampling. While adhesions from prior abdominal surgeries and anatomic abnormalities, such as large hiatal hernias, severe scoliosis, and previous bariatric procedures, may pose significant technical challenges for the procedure, these are not absolute contraindications for a surgeon with robotic experience. However, these factors should be considered strongly early in one’s experience.
Cross sectional imaging with multi-phase intravenous contrast such as helical computed tomography (CT) should be performed for all potential candidates. Contrast-enhanced magnetic resonance imaging (MRI) with- or without cholangiopancreatography (MRCP) is an acceptable alternative. These studies provide information on the nature of the primary pathology and its anatomic relationship to key surrounding structures such as the splenic vessels and celiac axis (17). Endoscopic ultrasound (EUS) is a useful adjunct to cross-sectional imaging allowing characterization of cystic lesions through aspiration and cyst fluid analysis (18). EUS-guided fine needle aspiration (FNA) may also allow tissue diagnosis, though some have speculated on a potential for seeding the transgastric needle tract (19). In our practice, the diagnostic information provided by trans-gastric FNA outweighs the theoretical risk of tumor seeding. For patients with suspicion of a neuroendocrine tumor octreotide scintigraphy, serum chromogranin A, and assessment of serum hormone concentrations [gastrin, insulin/pro-insulin/C-peptide, glucagon, vasoactive intestinal polypeptide (VIP), pancreatic polypeptide (PP), and 5-HT] may be indicated based on clinical suspicion and patient symptoms.
As with any major operation a thorough cardiopulmonary risk stratification and preoperative optimization is performed. Risk factors for complications following DP include male gender, age, high body mass index (20), soft pancreas (21), chronic pancreatitis (22), malnutrition (hypoalbuminemia), higher American Society of Anesthesiologists score (23), and smoking (24). Among these, smoking and malnutrition are the only modifiable risk factors. Significant weight loss (≥10% pre-morbid body weight), pancreatic insufficiency, biliary obstruction, new-onset or worsening diabetes mellitus, and poor alimentation are of particular importance.
In summary, an individualized and comprehensive approach to patient selection will maximize the chances of operative success while minimizing the chances of perioperative morbidity.
Pre-operative preparation
Bowel preparation is not routinely indicated. Patients adhere to a clear liquid diet for 24 h prior to operation and nil per os starting the midnight prior to surgery. This approach is modified for patients with insulinoma as they generally require admission to the hospital for intravenous 10% dextrose infusion and blood glucose monitoring. A prophylactic, intravenous, broad-spectrum antibiotic is routinely given within an hour of skin incision and re-dosed during operation according to Surgical Care Improvement Project (SCIP) guidelines. Deep vein thrombosis (DVT) prophylaxis with subcutaneous unfractionated or low molecular-weight heparin is also performed routinely. Epidural or para-vertebral regional analgesia is often a useful adjunct for post-operative pain relief.
Equipment preference card
Dissection is carried out with monopolar cautery hook dissector, fenestrated bipolar cautery grasper, and utility grasper forceps (ProGrasp™). A self-retaining liver retractor (Mediflex, Islandia, NY, USA), robotic scissors and Maryland dissectors are used as needed. Standard laparoscopic graspers, scissors, suction-irrigator, and a vessel sealing device such as LigaSure (Covidien-Medtronic, Minneapolis, MN, USA) are important tools for the bedside assistant. In addition, laparotomy trays should be immediately available inside the operation room should indications arise for conversion to open surgery. Commonly used disposables include surgical staplers, laparoscopic clip applier, silastic vessel loops (cut to 4 inches), umbilical tape (cut to 6 inches), and sutures.
Procedure
A typical operating room setup is depicted in Figure 2. The patient is positioned supine with the right arm tucked and the left arm extended at the shoulder on a split-leg table. Intravenous access, a nasogastric tube and Foley catheter are placed routinely while an arterial catheter is placed selectively as indicated. All pressure points are padded and we secure the patient to the operative table with straps and foot supports in order to prevent relative movement between patient and the robot (Figure 3).
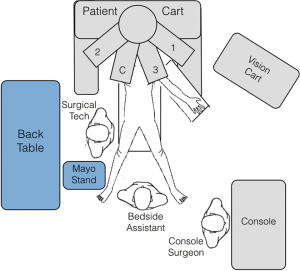
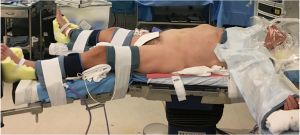
The general order or the operative steps outlined below are not significantly different from laparoscopic DP and they may be modified or re-arranged as clinically indicated.
Port placement/laparoscopy
With the patient in neutral position a 5-mm optical trocar is introduced through the rectus sheath slightly left of midline and 2–3 cm above the level of the umbilicus. Depending on the expected location of adhesions, the method of initial access (i.e., Hasson technique) may be adjusted. Once pneumoperitoneum is established, assess the peritoneum and abdominal organs for evidence of metastatic disease or other contraindications to resection. If none are found, additional trocars are placed as shown in Figure 4. Approximately 10 cm is required between trocars to minimize conflicts between robotic arms.

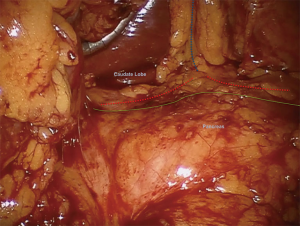
The dissection commences by entering the lesser sac through the gastrocolic ligament and opening it widely with a vessel-sealing device, taking care to preserve the gastroepiploic arcade and short gastric vessels (Figure 5). Laterally, the splenocolic ligament is divided and the splenic flexure of the colon is mobilized inferiorly. At this point the anterior surface of the pancreas is exposed by dissecting adhesions between the stomach and pancreas until the left gastric artery/vein pedicle can be visualized and the liver retractor can be positioned to elevate both the stomach and the left lateral segment of the liver anteriorly, against the abdominal wall (Figure 6). Intraoperative ultrasound is used to localize the target pathology, main pancreatic duct, splenic artery, common hepatic artery, superior mesentery artery and superior mesenteric vein.

Patient cart positioning and robot docking
Depending on the robotic system being used, the patient cart is positioned over the patient’s head (da Vinci S and Si) or from the patient’s right side (Xi). Robotic instruments include a ProGrasp™ forceps in arm 1, fenestrated bipolar cautery grasper in arm 2, and monopolar cautery hook dissector in arm 3 (Figure 4).
Dissection
The peritoneum along the inferior border of the pancreas is incised with monopolar hook cautery until the pancreatic body can be elevated and the splenic vein identified posteriorly. At the superior border of the pancreas, the splenic artery is identified and dissected circumferentially so that a vessel loop can be placed. Take care to avoid grasping the artery wall directly to prevent trauma or an intimal dissection flap(s) that can predispose to pseudoaneurysm.
The transection point on the pancreatic body is identified (visually or by ultrasound) and marked. A tunnel is created at the planned transection point between the posterior surface of the pancreas and the splenic vein or superior mesenteric vein (SMV) and an umbilical tape is placed to encircle the pancreas (Figure 7). Depending on the thickness of the pancreas, a laparoscopic stapler with 3–4-mm (purple load) or 4–5-mm (black load) staples (Endo GIATM, Medtronic, Minneapolis MN, USA) is used to divide the pancreatic body. A 15-mm trocar is required for black load staplers. Alternatively, the pancreas can be divided with cautery and the stump oversewn with Prolene mattress sutures.

Management of the distal pancreatic stump is controversial. Postoperative pancreatic fistula (POPF) at one week after stapler use was 32% in the multicenter European DISPACT trial, similar to the hand-sewn group (27). Additional staple-line enforcement with seamguard or fibrin glue (28,29) is not associated with decreased POPF rates in retrospective studies. A randomized, controlled trial failed to provide conclusive evidence due to poor accrual (30). With no consensus on the optimal stump closure, the method is determined by surgeon preference and individual patient factors. Our practice is to use surgical staplers without adjunctive measures unless clinical concern for inadequate stump closure is apparent in which case we oversew the stump with a Prolene mattress suture.
If a vessel-sacrificing approach (Warshaw) is chosen, the splenic artery and vein are divided near the pancreatic transection margin using separate vascular staple loads (Endo GIA tan load 2–3-mm staples). The distal pancreas is then mobilized in a medial-to-lateral fashion and the splenic vessels are divided again at the splenic hilum. If a vessel-sparing approach (Kimura procedure) is chosen, the distal portion of the pancreas is dissected from the splenic vein and tributary branches from the pancreas to the splenic vein are sequentially identified, isolated and divided using bipolar cautery, a vessel sealing device, or suture ligation (Figure 8). The pancreatic specimen is dissected from the splenic artery with a combination of monopolar cautery and vessel sealing device. Vascular sutures (4-0, 5-0 Prolene cut to 6 inches) should be immediately available throughout this phase of the procedure to manage inadvertent vessel injury.

Specimen extraction/drain placement
Once freed the pancreatic specimen is placed in a retrieval bag and delivered through the left lower quadrant incision which is enlarged transversely as needed. Frozen section analysis is performed to confirm negative margins. In order to re-insufflate the abdomen, we use the GelPOINT-Mini™ (Applied Medical, Rancho Santa Margarita, CA, USA). Alternatively, if one assistant port is sufficient for the remainder of the procedure the incision can be closed.
A 19-French Blake channel drain (Ethicon, Sommerville, NJ, USA) is routinely placed through the left upper quadrant robotic port next to the pancreatic stump. Following removal of all instruments under direct visualization, any fascial incisions greater than 8 mm are closed.
Post-operative management
Care following RADP is similar to laparoscopic DP. Intensive care unit admission is generally not indicated. Regional analgesia with epidural or para-vertebral catheters is continued postoperatively as are chemical and mechanical prophylaxis against DVT. The Foley catheter is removed postoperative day 1 or 2 and nasogastric tubes are usually removed on the day of surgery or postoperative day 1. Oral sips or a clear liquid diet is initiated on postoperative day 1 and advanced as tolerated to a regular diet.
The management of pancreatic drains is standardized and adapted from a protocol published by Molinari et al. (32,33). Briefly, serum and drain fluid amylase activity are assayed on the morning of postoperative day 3. If the drain fluid amylase activity is less than or equal to three-times the serum amylase activity and the patient is clinically well, the drain is removed on the following day regardless of the drainage volume.
Tips, tricks and pitfalls
The initial dissection is aimed at widely opening the lesser sac and freeing the posterior body and antrum of the stomach from the anterior surface of the pancreas allowing identification of the pillar of tissue containing the left gastric artery and vein with the caudate lobe to the right and diaphragmatic crus to the left (Figure 6). Visualizing this landmark allows identification of the common hepatic and splenic arteries near their origin at the celiac trunk. The splenic artery can be isolated, dissected and divided here (Warshaw technique). Complete mobilization of the splenic flexure of the colon is advisable both to improve visualization of the tail of the gland and to avoid injury to the colon or the tip of the spleen while retracting the colon caudally.
We routinely use intraoperative ultrasound to visualize the primary pathology and plan a transection point with an appropriate margin. Doppler flow ultrasound is also used to confirm pulsatile flow in the hepatic artery while transiently occluding the splenic artery.
Dissecting the pancreas from the splenic vein—if it is to be preserved—is a critical step. Inadvertent tearing of the bridging veins can result in significant blood loss and obscuring of the surgical field making controlled hemostasis difficult or impossible. Initially, direct pressure nearly always controls bleeding and more exact occlusion of the bleeding point can be performed with tissue forceps after clearing the field with suction. Hemostatic suture(s) (4-0 Prolene SH needle, 6 inches in length) can then be placed in a controlled manner. The splenic vein can be divided if bleeding is unable to be controlled with more conservative measures though this may require converting to a spleen-sacrificing procedure. Often this is preferable to converting to an open procedure.
When indicated, we create a vascularized tissue flap with the falciform ligament to protect the splenic artery stump. Anecdotally, this protects against pseudoaneurysm formation and may reduce the incidence of severe post-pancreatectomy hemorrhage.
Splenic infarction and/or abscess, though unusual (<5%) (32), may be a sequela of spleen-preserving DP. Intraoperative visual or Doppler assessment of spleen vascularity at the end of the dissection may help to determine those patients in whom the spleen is not salvageable. Others will present with pain and/or fevers postoperatively. If an undrained abscess is present, antibiotics along with percutaneous image-guided drainage is typically sufficient management. Otherwise, sterile ischemia or necrosis of the spleen may be managed with analgesics and supportive care. Re-operative splenectomy may be required for intractable pain.
Conclusions
The spleen-preserving RADP has demonstrated feasibility and safety in the hands of experienced robotic surgeons. As with all pancreatic surgery, standardization of care and a robust multidisciplinary clinical support team are keys to performing these complex procedures in a safe and efficient manner.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yoon YS, Lee KH, Han HS, et al. Patency of splenic vessels after laparoscopic spleen and splenic vessel-preserving distal pancreatectomy. Br J Surg 2009;96:633-40. [Crossref] [PubMed]
- Hwang HK, Chung YE, Kim KA, et al. Revisiting vascular patency after spleen-preserving laparoscopic distal pancreatectomy with conservation of splenic vessels. Surg Endosc 2012;26:1765-71. [Crossref] [PubMed]
- Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg 1999;229:693-8; discussion 698-700. [Crossref] [PubMed]
- Briggs CD, Mann CD, Irving GR, et al. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg 2009;13:1129-37. [Crossref] [PubMed]
- Suman P, Rutledge J, Yiengpruksawan A. Robotic distal pancreatectomy. JSLS 2013;17:627-35. [Crossref] [PubMed]
- Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554-9; discussion 559-62. [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. [Crossref] [PubMed]
- Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. [Crossref] [PubMed]
- Hwang HK, Kang CM, Chung YE, et al. Robot-assisted spleen-preserving distal pancreatectomy: a single surgeon's experiences and proposal of clinical application. Surg Endosc 2013;27:774-81. [Crossref] [PubMed]
- Ballantyne GH. Telerobotic gastrointestinal surgery: phase 2--safety and efficacy. Surg Endosc 2007;21:1054-62. [Crossref] [PubMed]
- Yu X, Li H, Jin C, et al. Splenic vessel preservation versus Warshaw's technique during spleen-preserving distal pancreatectomy: a meta-analysis and systematic review. Langenbecks Arch Surg 2015;400:183-91. [Crossref] [PubMed]
- Kimura W, Yano M, Sugawara S, et al. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein: techniques and its significance. J Hepatobiliary Pancreat Sci 2010;17:813-23. [Crossref] [PubMed]
- Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg 1988;123:550-3. [Crossref] [PubMed]
- Miura F, Takada T, Asano T, et al. Hemodynamic changes of splenogastric circulation after spleen-preserving pancreatectomy with excision of splenic artery and vein. Surgery 2005;138:518-22. [Crossref] [PubMed]
- Tien YW, Liu KL, Hu RH, et al. Risk of varices bleeding after spleen-preserving distal pancreatectomy with excision of splenic artery and vein. Ann Surg Oncol 2010;17:2193-8. [Crossref] [PubMed]
- Zhao WY, Luo M, Sun YW, et al. Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: a systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int 2009;8:457-64. [PubMed]
- Hunt GC, Faigel DO. Assessment of EUS for diagnosing, staging, and determining resectability of pancreatic cancer: a review. Gastrointest Endosc 2002;55:232-7. [Crossref] [PubMed]
- Beane JD, House MG, Coté GA, et al. Outcomes after preoperative endoscopic ultrasonography and biopsy in patients undergoing distal pancreatectomy. Surgery 2011;150:844-53. [Crossref] [PubMed]
- Ferrone CR, Warshaw AL, Rattner DW, et al. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg 2008;12:1691-7; discussion 1697-8.
- Eguchi H, Nagano H, Tanemura M, et al. A thick pancreas is a risk factor for pancreatic fistula after a distal pancreatectomy: selection of the closure technique according to the thickness. Dig Surg 2011;28:50-6. [Crossref] [PubMed]
- Distler M, Kersting S, Rückert F, et al. Chronic pancreatitis of the pancreatic remnant is an independent risk factor for pancreatic fistula after distal pancreatectomy. BMC Surg 2014;14:54. [Crossref] [PubMed]
- Goh BK, Tan YM, Chung YF, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg 2008;143:956-65. [Crossref] [PubMed]
- Nathan H, Cameron JL, Goodwin CR, et al. Risk factors for pancreatic leak after distal pancreatectomy. Ann Surg 2009;250:277-81. [Crossref] [PubMed]
- Juo YY, King JC. Opening of the lesser sac and exposure of pancreas. Asvide 2017;4:394. Available online: http://www.asvide.com/articles/1708
- Juo YY, King JC. Dissection of the pancreas and splenic artery, encircling and transection of pancreas. Asvide 2017;4:395. Available online: http://www.asvide.com/articles/1709
- Diener MK, Seiler CM, Rossion I, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 2011;377:1514-22. [Crossref] [PubMed]
- Jimenez RE, Mavanur A, Macaulay WP. Staple line reinforcement reduces postoperative pancreatic stump leak after distal pancreatectomy. J Gastrointest Surg 2007;11:345-9. [Crossref] [PubMed]
- Thaker RI, Matthews BD, Linehan DC, et al. Absorbable mesh reinforcement of a stapled pancreatic transection line reduces the leak rate with distal pancreatectomy. J Gastrointest Surg 2007;11:59-65. [Crossref] [PubMed]
- Shubert CR, Ferrone CR, Fernandez-Del Castillo C, et al. A multicenter randomized controlled trial comparing pancreatic leaks after TissueLink versus SEAMGUARD after distal pancreatectomy (PLATS) NCT01051856. J Surg Res 2016;206:32-40. [Crossref] [PubMed]
- Juo YY, King JC. Dissecting the pancreas from the splenic artery and vein and freeing the specimen. Asvide 2017;4:396. Available online: http://www.asvide.com/articles/1710
- Molinari E, Bassi C, Salvia R, et al. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg 2007;246:281-7. [Crossref] [PubMed]
- Bassi C, Molinari E, Malleo G, et al. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg 2010;252:207-14. [Crossref] [PubMed]
Cite this article as: Juo YY, King JC. Robotic-assisted spleen preserving distal pancreatectomy: a technical review. J Vis Surg 2017;3:139.


