VATS segmentectomy: an underused option?
Background
It is accepted that the “gold standard” procedure for surgically resectable early-stage non-small cell lung cancer (NSCLC) still remains pulmonary lobectomy, either with thoracotomy or thoracoscopic approach.
Such belief comes from multiple considerations: (I) the majority of thoracic surgeons are convinced that the lobectomy is “oncologically safer”, because the anatomical sub-unit represented by lobe has its own arteries, veins, bronchi and fissures; (II) the conviction that “we always performed this way”; (III) the results of the first randomized trial published in 1995 by Ginsberg et al. on behalf to the Lung Cancer Study Group (1). Such manuscript showed a high loco-regional recurrence rate (17.2% vs. 6.4%) and a worse survival in patients submitted to sublobar resections (SLR). This was the only randomized trial published at present that compared survival between sublobar resection with lobectomy in stage I NSCLC. Unfortunately, the study had strong limitations: “sublobar” resections included lung segmentectomies and wedge resections, many of the operators were general surgeons with limited thoracic experience, the sample size was underpowered, but, most importantly, T1 comprised tumors up to 3 cm in diameter. In addition, the conclusions of Ginsberg’s study have been confirmed in a non-randomized prospective trial published by Landreneau et al. in 1997 (2).
From 1995 to nowadays several articles have been published, but most of them are retrospective analysis. The results published were contradictory, depending to the point of view and the population analyzed, but most of them are in favour of segmentectomy in early stage NSCLC, in particular regarding the overall survival (OS) and cancer-specific survival.
The research question
To answer the question “Is VATS lung segmentectomy an under-used option?” the authors decided to look at the literature with a non-systematic review of what is published and what is in progress now.
Methods
The authors carried out a “four-step” non-systematic review in the field of sublobar pulmonary resections versus pulmonary lobectomies for early stage of lung cancer. The revision was conducted as follows:
- The authors made a non-systematic review, with MEDLINE as primary source. The search string was as follows: (((“lung”[All Fields] OR “pulmonary”[All Fields]) AND “segmentectomy”[All Fields]) AND “lobectomy”[All Fields]) AND “cancer”[All Fields]);
- The second part consisted in the analysis of review articles published about the comparison between SLR and lobectomy for early stage lung cancer;
- The third part was the overview of the ongoing studies registered on line;
- The final part is an analysis of the technical aspects of wedge resection and segmentectomy.
Results
Non-systematic review
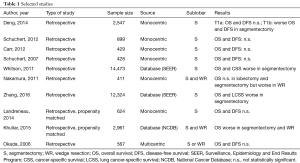
The results of the search have been filtered excluding papers with the following criteria: (I) survival data not reported; (II) comparison with lobectomy not reported; (III) case reports/case series; (IV) review paper; (V) expert opinions/editorials; (VI) non-English language. The papers found with the result search were 248. After a manually review of title and abstract and/or full text of each citation, the authors screened 40 papers. Of which, 10 papers were considered providing the “best evidence” in answering the authors’ question (Table 1).
Full table
Among retrospective studies, Deng et al. compared 212 segmentectomies with 2,336 lobectomies for stage I NSCLC: OS and disease-free survival (DFS) were not statistically different for T1a, but worse for T1b in segmentectomy (3). Other retrospective monocentric studies from USA published by Schuchert et al. and Carr et al. showed no differences in OS and DFS between segmentectomy and lobectomy for stage I NSCLC (4-6). Analyzing stages I to III NSCLC, Okumura et al. confirmed no differences in OS and DFS, with a long-term follow-up (7). On the contrary an important retrospective study published by Whitson et al. in 2011 showed OS and cancer-specific adjusted survival worse in segmentectomy than lobectomy for stage I NSCLC, analyzing a sample of 14,473 patients extracted from the Surveillance, Epidemiology, and End Results (SEER) Database (8). Nakamura et al. in a retrospective study compared wedge resection, segmentectomy and lobectomy for stage I NSCLC, revealing no differences between segmentectomy and lobectomy but worse 5-year OS in wedge resections (9). A Chinese work based on SEER Database enrolling more than 12,000 cases, found worse overall and lung cancer specific survival in segmentectomy compared to lobectomy in elderly patients (10).
Propensity matched studies included the Landreneau et al. paper, published in 2014, that showed the same OS and DFS in an enrolling 312 segmentectomies and 312 lobectomies (11). In 2015, an analysis from National Cancer Database (NCDB) was published by Khullar et al. comparing wedge, segmentectomy and lobectomy for pT1aN0 NSCLC with the propensity match method. The results reported in this paper established worse OS in wedge resections and segmentectomies compared with lobectomies (12).
Ongoing studies
Searching for registered ongoing studies, we found 6 trials based on different subsets of patients. In particular, four trials were registered from Chinese researchers: (I) “Comparison of Different Types of Surgery in Treating Patients with Early-stage Non-small Cell Lung Cancer” is a prospective, matched, controlled, open, multicenter trial. Patients with NSCLC <2 cm are matched according to age and ground-glass opacity (GGO) component ratio. Among SLR, wedge resection is permitted. The outcome of this non-randomized trial is the DFS but the planned 630 patients appears to be an underpowered sample size. (II) “Comparison of cVATS Segmentectomy Versus Lobectomy for Lung Adenocarcinoma in Situ and With Microinvasion” is a prospective, open-label, parallel, multi-center, randomized trial recruiting patients with <2 cm pure ground-glass or mixed ground-glass nodules (solid areas <0.5 cm). Considerations on sample size are similar to those expressed for the previous trial. (III) “Comparison of Lobectomy and Segmentectomy for cT1aN0M0 Peripheral NSCLC” is a randomized, parallel, open label trial with DFS as end-point. Even in this case the sample size (610 patients) looks as underpowered. (IV) “Surgical Treatment of Elderly Patients with cT1N0M0 Non-small Cell Lung Cancer Comparison between Sublobar Resection and Lobectomy (STEPS)” is a randomized, parallel, open label trial recruiting patients (≥70 years old) with ≤2 cm NSCLC; DFS is the end-point. The sample size is limited to 339 patients.
A multi-continental study (USA, Canada and Australia), enrolling an estimate number of 1,288 adult patients affected by stage I NSCLC, is aimed to compare lobectomy with SLR. The primary end-point of this study is DFS; a concern on sample size adequacy is present considering that wedge resections are permitted among the SLR.
Finally, a multicenter Japanese trial named “A Phase III Randomized Trial of Lobectomy Versus Limited Resection for Small-sized Peripheral Non-small Cell Lung Cancer” is recruiting patients with ≤2 cm peripheral NSCLC (13). Pulmonary lobectomy is the standard treatment and segmentectomy is the experimental one. Unlike previous studies, this trial has OS as primary end-point; this detail could make the sample size questionable.
The scientific community is looking forward to having the results from the mentioned studies but it is notable the absence of any European project.
Technical aspects
SLR include various types of limited excision of parenchyma, with considerable differences from a technical, anatomical and oncological point of view.
Depending from the hilum dissection, SLR are divided into non-anatomic (wedge resections) and anatomic (segmentectomies).
A wedge resection is the removal of a triangle-shaped slice of tissue, usually for small peripheral lung lesions, without dissection of pulmonary hilum. This makes the procedure technically simple, even in VATS. On the other hand, the distance between the nodule and the surgical margin is usually inferior to the anatomic procedures, as well as the assessment of hilar lymph nodes, which is frequently limited.
An anatomical segmentectomy is the removal of one or more pulmonary segments, after individual dissection of vein, artery and bronchus. After division of hilar structures, intersegmental plan is identified, to guide the parenchymal section strictly to the proper segment to be removed.
Segmentectomy allows a wider parenchymal resection than wedge resections, with a lower risk of inadequate surgical margins. Moreover, segment specific lymphatics are removed, ensuring a valid radicality in early stage NSCLC. The total number of resected lymph nodes is significantly higher than in wedge resections, as reported in several studies (14-16).
Hilum dissection in anatomical segmentectomies can be technically more complex than a lobectomy, because of the extended and more distal isolation of segmental vessels and bronchi.
Moreover, some segments can be difficult to be removed for the very deep location of vessels and bronchi into the parenchyma (i.e., dorsal and lateral basal segments of lower lobes). For this reason, the majority of segmentectomies performed are left upper lobe trisegmentectomy, lingulectomy, and apical segmentectomy of lower lobes.
In case of suspect hilar adenopathy, it’s mandatory to have a frozen section on segment-specific lymph nodes: if the response is positive, we don’t consider segmentectomy radical from an oncological point of view. In that case, if the patient’s respiratory function is permissive, the strategy change in favor of lobectomy is advisable.
After hilum division, intersegmental plane can be challenging to be identified. Usually we ask to the anesthesiologist to inflate the lung before segmental bronchial closure, keeping the proper bronchus closed with a clamp. This helps to identify the limit between the involved segment, that remains deflated, and the parenchyma to be preserved. If collateral ventilation is relevant, the intersegmental plane can be difficult to be identified.
Other techniques for distinguishing the intersegmental plane have been proposed. Tsubota in 2000 described the inflation of the lobe followed by the bronchus closure, in order to maintain gas inside the segment to be removed (17). Okada in 2007 described the application of jet ventilation under bronchoscopic guide to cause selective segmental inflation (18). Sekine in 2012 reported the transbronchial injection of indocyanine green, followed by infrared thoracoscopy (19). Zhang in 2015 described a staining technique with methylene blue injection into the segmental bronchus (20).
After intersegmental plane identification, parenchyma division is usually performed with stapler, as in our centre. Some authors reported use of cautery cutting (18) or energy based devices. In case of thick lung resection, autologous or synthetic tissue reinforce on the staple line can be useful to prevent air leakage.
Although technically demanding, most of anatomic segmentectomy are now performed with VATS or RATS. We believe the mini-invasive procedure is feasible and safe, even in uniportal VATS, if surgeon’s experience is adequate (21,22).
The Italian point of view
Looking at the Italian situation, analyzing the case series reported and papers published, attending National meetings on Thoracic Surgery and speaking with colleagues, the feed-back is that several Thoracic Surgery Departments sometimes carried out segmentectomy in early stage NSCLC, but without a precise rule. In fact, often segmentectomy is proposed to patients that cannot be undertaken to lobectomy because of age, poor cardiopulmonary function or relevant comorbidities, and previous thoracic surgery, especially for recurrent tumors. Therefore, as mentioned before, the choice to decide for segmentectomy, either by thoracotomy or by VATS, is a “non-intentional” one.
Regarding the “non-intentional” choice of sublobar resection, Fiorelli et al. published a multicenter retrospective propensity matched analysis comparing sublobar resection (comprehending both wedge resection and segmentectomy) with lobectomy in elderly patients. The results showed no differences in long term survival between two populations (23).
In 2004 Campione et al. published a retrospective analysis where results showed no differences in survival but high incidence of local recurrence in segmentectomy compared to lobectomy for stage Ia NSCLC (24).
The Padua group, concerning surgical treatment of second primary cancers, concludes that lobectomy should be considered the treatment of choice, but sublobar resection remains a valid option in high-risk patients (25). In addition, Pardolesi et al. published in 2012 their initial experience about robotic segmentectomy, analyzing surgery outcome of 17 lung segmentectomies performed in two different centers. The authors concluded that robotic lung segmentectomy is feasible and safe (26).
Discussion
Interest in sub-lobar resections has been growing in recent years. Some authors have tried to summarize the evidence in the literature: Cao et al. published a review and meta-analysis in 2015; the results emphasized the patient selection between “intentionally selected” or “compromised” patients, and suggested segmentectomy as a feasible alternative to lobectomy in selected patients with peripherally <2 cm tumors with favorable histopathology and with ground glass imaging (27). On the contrary, a review published by Sihoe and Van Schil in 2014, pointed out that evidences regarding survival outcomes between “intentional” segmentectomy and lobectomy were too low; therefore, waiting ongoing randomized trials results, they considered lobectomy as standard therapy for early stage NSCLC (28). A meta-analysis published by Bao et al. in 2014 suggest that segmentectomy provides worse survival than lobectomy in NSCLC from 2 to 3 cm of diameter, but equivalent survival in tumors smaller than 2 cm (29).
Completing our multi-step narrative revision, we observed a lack of evidence about the oncological value of segmentectomy in early stage NSCLC. In fact, several authors published papers with contradictory results; this is not surprising considering that almost all of the papers were retrospective cohort studies with limited population and that studies based on large national database frequently suffer from incomplete record. Data that are difficult to find in retrospective studies are why and when the sublobar resection has been performed. In other words, segmentectomy or wedge resection can be “intentional” or “not intentional”. An “intentional” sublobar resection is done in patients that could tolerate a major resection (i.e., lobectomy). If the sublobar resection was “not intentional”, probably the parenchyma sparing procedure was chosen due to poor postoperative pulmonary and/or cardiac function or other reasons that inevitably could impact on survival. Some retrospective studies were propensity matched, that adds statistical value to the results, but unfortunately, they were not powered enough to analyzing a survival-based outcome.
The lack of randomized prospective studies is probably due to different reasons: firstly, the “ethical problem” for randomization: in fact it’s quite difficult to propose a segmentectomy to a fitting patient, that can tolerate a lobectomy; secondly, proper sample size for a non-inferiority study on this issue requires thousands of patients; thirdly, a large scale multicenter randomized study has important economic and organizing impact. Nevertheless, both in Asia and North America, prospective randomized studies are ongoing; a European study would be desirable for the racial and socio-economic diversity of the Old Continent.
Certainly SLR, particularly segmentectomy, could be attractive for many reasons. Firstly, the growing incidence of GGO tumors, especially in Asiatic people; the main features of these tumors are to be “multi-focal” and have low rate of local recurrences, that potentially require multi-step or redo surgery (as “intentional” procedures). Secondly the rising in expectancy of life requires lung resections in elderly patients who could benefit from parenchyma-sparing, “non-intentional” procedures; thirdly, the ongoing lung cancer screening programs allow detections of more early-stage lung opacities in high-risk patients, who, once more, can functionally benefit from “not intentional” segmentectomy.
Although VATS segmentectomy is performed by experienced thoracic surgeons, it’s not yet world-widely adopted. The main technical obstacle is the long learning curve of VATS segmentectomy and the difficulty to resect all pulmonary segments.
Of course, the interest in this field is increasing, both from oncological and technical point of view.
To conclude, our impression is that segmentectomy is mainly adopted as “not intentional” procedure, in high risk patients who cannot tolerate lobectomy. The surgical scientific community is looking forward to receiving the results of ongoing and future randomized trials, that could answer to our dilemma: “to do or not to do ‘intentional’ segmentectomy in early NSCLC?” In the meantime, we are confident that VATS segmentectomy could become widely adopted among thoracic surgeons.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resections for T1N0 non-small cell lung cancer. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resections versus lobectomy for stage I (T1N0M0) non-small cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691-8; discussion 698-700. [Crossref] [PubMed]
- Deng B, Cassivi SD, de Andrade M, et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg 2014;148:1186-92.e3. [Crossref] [PubMed]
- Schuchert MJ, Awais O, Abbas G, et al. Influence of age and IB status after resection of node-negative non-small cell lung cancer. Ann Thorac Surg 2012;93:929-35. [Crossref] [PubMed]
- Carr SR, Schuchert MJ, Pennathur A, et al. Impact of tumor size on outcomes after anatomic lung resection for stage 1A non-small cell lung cancer based on the current staging system. J Thorac Cardiovasc Surg 2012;143:390-7. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32. [Crossref] [PubMed]
- Okumura M, Goto M, Ideguchi K, et al. Factors associated with outcome of segmentectomy for non-small cell lung cancer: long-term follow-up study at a single institution in Japan. Lung Cancer 2007;58:231-7. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2011;59:137-41. [Crossref] [PubMed]
- Zhang Y, Yuan C, Zhang Y, et al. Survival following segmentectomy or lobectomy in elderly patients with early stage lung cancer. Oncotarget 2016;7:19081-6. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and serviva outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62. [Crossref] [PubMed]
- Speicher PJ, Gu L, Gulack BC, et al. Sublobar resection for clinical Stage IA non-small-cell lung cancer in the United States. Clin Lung Cancer 2016;17:47-55. [Crossref] [PubMed]
- Tsubota N. An improved method for distinguishing the intersegmental plane of the lung. Surg Today 2000;30:963-4. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Zhang Z, Liao Y, Ai B, et al. Methylene blue staining: a new technique for identifying intersegmental planes in anatomic segmentectomy. Ann Thorac Surg 2015;99:238-42. [Crossref] [PubMed]
- Gonzalez-Rivas D, Mendez L, Delgado M, et al. Uniportal video-assisted thoracoscopic anatomic segmentectomy. J Thorac Dis 2013;5 Suppl 3:S226-33. [PubMed]
- White A, Swanson SJ. Video-assisted thoracic surgery (VATS) segmentectomy: state of the art. Minerva Chir 2016;71:61-6. [PubMed]
- Fiorelli A, Caronia FP, Daddi N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today 2016;46:1370-82. [Crossref] [PubMed]
- Campione A, Ligabue T, Luzzi L, et al. Comparison between segmentectomy and larger resection of stage IA non-small cell lung carcinoma. J Cardiovasc Surg (Torino) 2004;45:67-70. [PubMed]
- Zuin A, Andriolo LG, Marulli G, et al. Is lobectomy really more effective than sublobar resection in the treatment of second primary lung cancer? Eur J Cardiothorac Surg 2013;44:e120-5. [Crossref] [PubMed]
- Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. [Crossref] [PubMed]
- Cao C, Chandakumar D, Gupta S, et al. Could less be more? – A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selections. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014;86:115-20. [Crossref] [PubMed]
- Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg 2014;46:1-7. [Crossref] [PubMed]
Cite this article as: Mendogni P, Tosi D, Rosso L, Palleschi A, Cattaneo M, Mazzucco A, Nosotti M. VATS segmentectomy: an underused option? J Vis Surg 2017;3:136.


