Uniportal video-assisted thoracoscopic surgery in hemothorax
Introduction
Hemothorax is defined as a collection of fluid in the pleural cavity with a hematocrit greater than 25–50% of patient’s blood and its aetiology encounters thoracic trauma, coagulation disorders or iatrogenic mechanisms (e.g., central line insertion, thoracentesis, pleural biopsies or cardio-thoraco-abdominal surgical interventions). Spontaneous hemothorax is a very rare clinical scenario and it is due to concomitant pneumothorax, to vascular disorders, connective tissue disease, neoplasms, extramedullary haematopoiesis, endometriosis or pulmonary sequestration (1-4).
In the daily practice, the most common cause of hemothorax is trauma and these cases are conservatively managed (pain control, chest drainage, physiotherapy, prophylactic antibiotics) and only a very limited number of patients are considered for an emergency thoracotomy, usually for life-threatening injuries (less than 10%) (5).
Despite studies showing excellent results with the “chest drainage only” treatment, in the vast majority of cases the clinical improvement is more apparent than real: in fact, in 5–30% of cases the hemothorax does not reabsorb (6,7), in 24% a pleural empyema raises (8) and in up to 39% fibrothorax and pulmonary trapping associated with various degree of respiratory failure develop (6).
In the early 1990s, the raise of video-assisted thoracic surgery (VATS) offered to thoracic surgeons the proper tool to tackle the retained or post-operative hemothorax, with lower morbidity due to minimized surgical incisions, better visualization, a higher yield of detecting small injuries, less post-operative pain, improved lung function, shorter hospital stay and earlier recovery (2,5,7,8).
More recently, the uniportal VATS approach has gained popularity in thoracic surgery, initially for minor procedures (9,10) and then also for major lung resection (11,12). This paper summarised recent advances available in the medical literature focusing especially on the VATS treatment of hemothorax, in particular considering the advantages of uniportal versus multiportal approach.
Multiportal VATS strategy: basic concepts
Thoracoscopy in the last years has been suggested as the first-line treatment in non-complicated hemothorax, leaving thoracotomy as the rescue option in case of failure or major trauma with bleeding (7). Truly, Joao Martins Castello Branco in 1946 was the first to describe the use of thoracoscopy in thoracic trauma, managing persistent bleeding with electro-coagulation. Since then, many authors described the use of this technique for diagnostic (diaphragmatic lesions) and therapeutic (hemothorax in penetrating chest trauma) options (13,14). However, only in the late 1990s, due to technical advances, its use became worldwide popular and VATS gained a primary position in the care of thoracic trauma patients (7,15). Villavicencio et al. (16) demonstrated that thoracoscopy may be used safely and successfully in assessment of clotted or persistent hemothorax and in empyema drainage; according to their data, 90–95% of patients with post-traumatic hemothorax are treated conservatively or with tube thoracostomy only, 20% develop later a clotted or a persistent loculated hemothorax after drainage and 40% of them require eventually a surgical procedure.
According to Helling et al. (6), VATS has a definitive place in the early phase of management, especially when the blood loss is 100 mL/h or more after 48 h and clots have not been entirely evacuated by chest tube. The goals of this upfront approach are multiple: (I) the quick and early evacuation of clots reduces the risk of fibrinolysis activation with maintenance of bleeding; (II) better chest tube positioning; (III) direct treatment of injuries responsible of bleeding; (IV) avoidance of further and multiple (unnecessary transfusions); (V) shorter hospital stay and hence reduced healthcare costs.
The technical details of the procedure have been summarized by Fabbrucci et al.: under general anaesthesia and with double lumen intubation, the first incision is made on the site of the thoracostomy tube and this port is used to introduce the scope. The site of the further ports (2-3-4 ports in any case) is determined from inside after the initial evaluation of the chest cavity, of the amount of blood/clots and of the bleeding source (15).
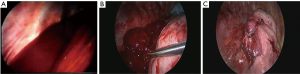
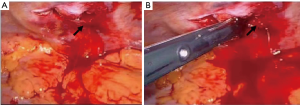
The aetiology of the hemothorax plays a great role in the surgical strategy. In most of the cases of post-traumatic retained hemothorax, clots removal, copious irrigation of the cavity with partial decortication of the lung are safe and adequate for the final success of the procedure (Figure 1). On the contrary, in penetrating injuries, it is mandatory to accurately check the lung parenchyma and the chest wall to identify the source of bleeding (Figure 2A) and control it safely (Figure 2B).
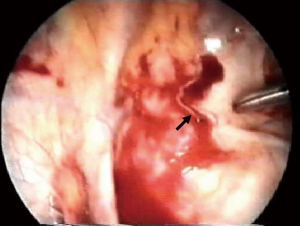
In the cases of spontaneous hemothorax, it is mandatory to explore the pericardium, the pleural cavity and the chest wall to find vascularised pleural adherences (Figure 3), osteochondral exostosis (Figure 4A,B) or neoplastic wall implants that cause the onset and maintenance of the hemothorax (1,2).
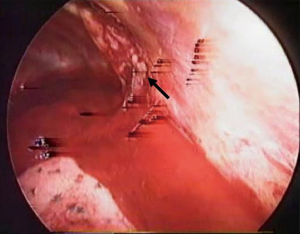
Iatrogenic post-operative hemothorax could be initially managed thoracoscopically, if the patient is hemodynamically stable (2,3,7). The use of chest drain orifices or part of the thoracotomy is useful for the initial assessment of the pleural cavity, being the intercostals vessels or the thoracotomy line often the cause of the bleeding, as in this case of post-operative bleeding from thoracotomic site (Figure 5).
The topic of time between diagnosis of the hemothorax and thoracoscopic drainage, probably affecting lowest complications rate and better outcomes, has not been fully clarified in the literature. Various studies showed that early evacuation results in less complications: but, while some authors (17,18) recommend the surgical procedure performed within 3 days following the diagnosis, others report successful outcomes in trauma patients when this is accomplished after 7 or more days (19,20). Villegas et al. in their series of 139 patients showed that the longer the time span between the moment of the trauma and the procedure, the higher the risk of complications and the conversion rate (21). Morales Uribe et al. on the basis of their experience concluded that a surgical procedure delayed after the 5th post-trauma day is associated with conversion to open surgery in 15.8% of cases or with the need of redo surgery, due to the presence of pleural thickening and dense adhesions between lung and chest wall (7). Ahmad et al. demonstrated that the outcome of VATS in retained hemothorax is directly dependent on the timing of intervention, with a conversion rate diminished from 15.8% to 7.7% if surgery is completed by the 6th post-traumatic day (5).
Uniportal VATS
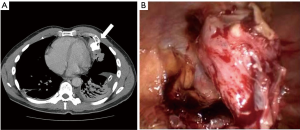
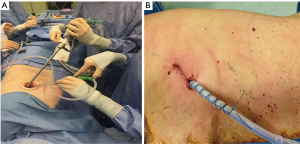
The uni-VATS was firstly proposed by Migliore et al. (9,10) and then mostly expanded by Rocco et al. (12). In his 10-year large experience of 644 patients, the evacuation of hemothorax was a very small part (2.3%) of his series. He reproduced single-port proposal (9), describing the method as an ideal way to reproduce the open approach to the chest. Through a single port, the fulcrum is moved to inside the chest through the introduction of articulating instruments, to avoid mutual interference. The difference between this technique and the standard 3-port VATS is that it develops along a sagittal plane rather than a latero-lateral one. The surgeon is located in front of the patient and the assistant can be located either in front or alongside of the surgeon (22). A single incision of 1.5–2.5 cm long in the 4th–6th intercostals space along the posterior axillary line (Figure 6A) was the initial standard approach, without further dissection of intercostal space or retractors. Indeed, the placement of the incision is of paramount importance to have a successful procedure and a learning curve is needed to obtain the best results. In the literature, uniportal VATS has also been described through subxiphoid, transaxillary, transsternal, transdiaphragmatic and transcervical approaches (23,24). Mandatory for a good technique is the use of adequate optics and articulated instruments, straight or curved, but flexible. The surgical technique is the same of multiportal VATS but with a single-port incision, with evacuation of clots, control of the bleeding points and correct tube placement, through the single incision (Figure 6B). A new frontier will be the performing of uniportal VATS on awake or sedated patients. From 2011 a second milestone in the use of uniportal VATS was done, the first uniportal VATS lobectomy was performed by Gonzales Rivas et al. (25).
Many authors have directed their work toward the difference in post-operative outcomes between uniportal VATS and multiportal VATS (11,12,26,27). All the authors agree that there were no differences between the two techniques about morbidity and mortality, association with cardiopulmonary complications, atrial fibrillation, atelectasis or wound infection, complications higher in the thoracotomic approach (11,12,26). The most important goal of uniportal VATS is the decrease of post-operative pain due to a single incision without rib spreading. Regarding the outcome of uniportal VATS compared with that of multiportal VATS, there are important conflicting opinions. Harris et al. (28) reviewing eight large retrospective studies on all types of VATS resections observed a shorter duration of chest tube and lower morbidity in uniportal VATS; Dai et al. (29) showed a reduction of intraoperative blood loss and post-operative pain; Chung et al. (30) saw no differences between the two techniques. The main accepted goal of uniportal VATS are the decreased pain and paresthesia, due to use of a single incision with less trauma on only one intercostals space and so only one intercostals nerve is likely to be stretched with a single distribution of pain (12). Jutley et al. (31) reported in a study about VATS treatment of pleural that the uniportal group has lower median pain score on the visual analogue scale if compared with 3-port VATS and 86% of uniportal VATS patients reported no long-term neurologic symptoms, while in the 3-port group only 42% of patients were asymptomatic. Tamura et al. (32) presented the same results in their series of 37 patients as like as Mier et al. (33) showed in a 20-patient series as the mean visual analogue scale pain score is significantly better for patients undergoing single incision VATS.
Conclusions
In conclusion, post-traumatic or spontaneous hemothorax in most cases require a conservative management with only chest tube insertion. When a retained hemothorax or persistent bleeding are present and the patient is hemodynamically stable, VATS approach is highly effective, particularly if early performed (within 5 to 7 days after trauma) and it is associated with low morbidity.
In this setting, the uniportal VATS approach offers a valid alternative to conventional multiport VATS technique and might potentially impact on post-operative pain, long-term pain and paresthesia, shorter hospital stay and rapid return to work for the patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Patrini D, Panagiotopoulos N, Pararajasingham J, et al. Etiology and management of spontaneous haemothorax. J Thorac Dis 2015;7:520-6. [PubMed]
- Samra SS, Samra NS, Jain A, et al. Video-assisted thoracic surgery for hemothorax following coronary artery bypass. Asian Cardiovasc Thorac Ann 2006;14:e19-20. [Crossref] [PubMed]
- Lin CY, Chang CC, Chuang MT. Spontaneous Haemothorax Secondary to Rib Exostosis. Heart Lung Circ 2017;26:e62-3. [Crossref] [PubMed]
- Okubo Y, Hamakawa H, Ueda H, et al. Extralobar Sequestration Presenting as Sudden Chest Pain Due to Hemothorax. Ann Thorac Surg 2016;101:e27. [Crossref] [PubMed]
- Ahmad T, Ahmed SW, Soomro NH, et al. Thoracoscopic evacuation of retained post-traumatic hemothorax. J Coll Physicians Surg Pak 2013;23:234-6. [PubMed]
- Helling TS, Gyles NR 3rd, Eisenstein CL, et al. Complications following blunt and penetrating injuries in 216 victims of chest trauma requiring tube thoracostomy. J Trauma 1989;29:1367-70. [Crossref] [PubMed]
- Morales Uribe CH, Villegas Lanau MI, Petro Sánchez RD. Best timing for thoracoscopic evacuation of retained post-traumatic hemothorax. Surg Endosc 2008;22:91-5. [Crossref] [PubMed]
- Cetindag IB, Neideen T, Hazelrigg SR. Video-assisted thoracic surgical applications in thoracic trauma. Thorac Surg Clin 2007;17:73-9. [Crossref] [PubMed]
- Migliore M, Giuliano R, Deodato G. Video assisted thoracic surgery through a single port. Proceedings of Thoracic Surgery and Interdisciplinary Symposium on the threshold of the third Millennium; 2000 May 11-13; Naples, IT: 2000;200:29-30.
- Migliore M, Deodato G. A single-trocar technique for minimally-invasive surgery of the chest. Surg Endosc 2001;15:899-901. [Crossref] [PubMed]
- Gonzalez-Rivas D. Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg 2016;5:85-91. [Crossref] [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [Crossref] [PubMed]
- Jackson AM, Ferreira AA. Thoracoscopy as an aid to the diagnosis of diaphragmatic injury in penetrating wounds of the left lower chest: a preliminary report. Injury 1976;7:213-7. [Crossref] [PubMed]
- Jones JW, Kitahama A, Webb WR, et al. Emergency thoracoscopy: a logical approach to chest trauma management. J Trauma 1981;21:280-4. [Crossref] [PubMed]
- Fabbrucci P, Nocentini L, Secci S, et al. Video-assisted thoracoscopy in the early diagnosis and management of post-traumatic pneumothorax and hemothorax. Surg Endosc 2008;22:1227-31. [Crossref] [PubMed]
- Villavicencio RT, Aucar JA, Wall MJ Jr. Analysis of thoracoscopy in trauma. Surg Endosc 1999;13:3-9. [Crossref] [PubMed]
- Milfeld DJ, Mattox KL, Beall AC Jr. Early evacuation of clotted hemothorax. Am J Surg 1978;136:686-92. [Crossref] [PubMed]
- Vassiliu P, Velmahos GC, Toutouzas KG. Timing, safety, and efficacy of thoracoscopic evacuation of undrained post-traumatic hemothorax. Am Surg 2001;67:1165-9. [PubMed]
- Lang-Lazdunski L, Mouroux J, Pons F, et al. Role of videothoracoscopy in chest trauma. Ann Thorac Surg 1997;63:327-33. [Crossref] [PubMed]
- Heniford BT, Carrillo EH, Spain DA, et al. The role of thoracoscopy in the management of retained thoracic collections after trauma. Ann Thorac Surg 1997;63:940-3. [Crossref] [PubMed]
- Villegas MI, Morales CH. Tratamiento del hemotórax coagulado mediante toracoscopia. Rev Col Cirugía 2000;15:29-31.
- Bertolaccini L, Rocco G, Viti A, et al. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5 Suppl 3:S214-6. [PubMed]
- Suda T, Ashikari S, Tochii S, et al. Single-incision subxiphoid approach for bilateral metastasectomy. Ann Thorac Surg 2014;97:718-9. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxyphoid single-incision thoracoscopic pulmonary metastasectomy. Thorac Cancer 2015;6:230-2. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Pastina M, Menna C, Andreetti C, et al. The era of uniportal video-assisted thoracoscopic surgery. J Thorac Dis 2017;9:462-5. [Crossref] [PubMed]
- Reinersman JM, Passera E, Rocco G. Overview of uniportal video-assisted thoracic surgery (VATS): past and present. Ann Cardiothorac Surg 2016;5:112-7. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Dai F, Meng S, Mei L, et al. Single-port video-assisted thoracic surgery in the treatment of non-small cell lung cancer: a propensity-matched comparative analysis. J Thorac Dis 2016;8:2872-8. [Crossref] [PubMed]
- Chung JH, Choi YS, Cho JH, et al. Uniportal video-assisted thoracoscopic lobectomy: an alternative to conventional thoracoscopic lobectomy in lung cancer surgery? Interact Cardiovasc Thorac Surg 2015;20:813-9. [Crossref] [PubMed]
- Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. [Crossref] [PubMed]
- Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: retrospective analysis between single-incision and three-port video-assisted thoracoscopic surgery. J Cardiothorac Surg 2013;8:153. [Crossref] [PubMed]
- Mier JM, Chavarin A, Izquierdo-Vidal C, et al. A prospective study comparing three-port video-assisted thoracoscopy with the single-incision laparoscopic surgery (SILS) port and instruments for the video thoracoscopic approach: a pilot study. Surg Endosc 2013;27:2557-60. [Crossref] [PubMed]
Cite this article as: Sanna S, Bertolaccini L, Brandolini J, Argnani D, Mengozzi M, Pardolesi A, Solli P. Uniportal video-assisted thoracoscopic surgery in hemothorax. J Vis Surg 2017;3:126.


