The current state of per oral endoscopic myotomy for achalasia
Introduction
Achalasia is an acquired neural degenerative disorder of the esophagus characterized by the presence of ineffectual or absent esophageal peristalsis and by the inability of the lower esophageal sphincter (LES) to relax. Patients with achalasia will typically present with symptoms of progressive dysphagia to solids and liquids. With failure to pass into the stomach, the patient may also experience regurgitation and/or aspiration type symptoms along with chest pain due to spasm of the esophageal muscle contracting against the closed sphincter. Treatment of achalasia has always been palliative and has been directed solely at the muscular anatomy of the LES rather than the underlying neuromuscular disorder. Disruption of the muscular fibers of the LES with a whalebone was the first effective method of improving the patient’s symptoms (1).
Although medical therapy is available, it rarely has had any durable effect. Current effective therapies all have one thing in common they lead to disruption of the LES. Traditionally, surgical myotomy as outlined originally by Heller (2), modified by Ellis (3) and then by Pellegrini (4) has been the mainstay of therapy even as the operation has become less and less invasive. Even a recent challenge by endoscopic pneumatic dilation has not resulted in wide spread adoption of this option at least in North America (5). However, with improvements in flexible endoscopy and endoscopic tools combined with the knowledge that the submucosal space can be used as access to the LES, a true endoscopic treatment for achalasia has been achieved.
In this review, we describe the development of per oral endoscopic myotomy (POEM) and its use for the treatment of achalasia including the current indications and contraindications. The technique and options are described in detail and the outcomes of the procedure on its own and in comparison with other treatments for achalasia.
Toward an endoscopic myotomy
The pursuit for a less invasive approach to surgical myotomy has been ongoing since Dr. Heller first published his series on the operation that bears his name (6). With the thoracic approach popularized by Dr. Ellis as the option to balance improvements in dysphagia whilst avoiding the development of GERD (7), the initial minimally invasive approach was thoracoscopic (8). But, this quickly gave way to the laparoscopic approach which has been considered the gold standard for almost 20 years (9,10).
Although POEM was introduced and popularized by Inoue and colleagues starting with their landmark report in 2010 (11), the intent of an endoscopic myotomy began in the 1980’s when Ortega et al. (12), motivated to avoid thoracotomy for open Heller myotomy and the complications of forceful pneumatic dilation began experimental work using a customized electrosurgical knife to perform an endoscopic transmucosal myotomy on dogs. They then expanded upon that initial work applying the same technique on seventeen humans with achalasia. Even though this procedure resulted in encouraging results with palliation in dysphagia, there were no further reports or follow up studies arising from this initial report.
After a 30-year hiatus, two important innovations were recognized. First, the concept of submucosal access to reach the muscular layer (13) and second, the safety of the mucosal flap created during tunneling through the submucosal space (14). To create access in the submucosal tunnel, a biliary balloon was used to help dissect open the submucosa. They recognized the value of the mucosal flap created by the tunnel and offsetting the entry point (mucosotomy) with the myotomy to protect the mediastinum. Building on this report, Inoue and colleagues, applied the techniques learned through endoscopic submucosal dissection to create the submucosal tunnel without the need for balloon dilation and using direct vision to dissect the space, coagulate any vessels and maintain orientation. Additionally, they introduced new endoscopic “knives” to provide accurate division of the muscular fibers and recommended the use of CO2 insufflation as a safer method of maintaining the space.
Patients for POEM
Currently, POEM is indicated in all patients with symptomatic achalasia of all types. Initially use of POEM was restricted to patients ≥18 years old, but it has now been used successfully in patients as young as 3 years and with no upper age limit, only comorbid conditions preventing general endotracheal tube anesthesia (15,16). POEM has also been utilized for treating a variety of esophageal motility disorders in patients with a wide age range (17).
There are few contraindications to POEM outside of serious systemic illness. However, caution is raised in patients who have undergone prior treatments that might obliterate the submucosal plane such as a prior perforation that was repaired. Additionally, patients with a known hiatal hernia should be counseled that a laparoscopic approach may be reduce the risk of refractory reflux since the hernia can be repaired. Relative contraindications to the procedure include: prior irradiation to the mediastinum or esophagus, severe pulmonary disease, coagulopathy with thrombocytopenia under 50,000, history of prior esophageal mucosal resection, compensated cirrhosis with portal hypertension (17). The procedure is available to patients with prior pneumatic dilation, Heller myotomy, or POEM. The only absolute contraindications have recently been limited to the inability to tolerate general anesthesia and being unable to safely stop anticoagulation prior to the procedure (18).
Pre- and intraoperative considerations
In preparation for POEM, patients are put on a full liquid diet for 3 days leading up to the procedure and changed to clear liquids for 1 day before surgery to clear the abnormally emptying esophagus from residual food particles (17). Nystatin 500,000 IU four times per day is given for 3 days prior to surgery. Intravenous proton pump inhibitors are typically given preoperatively due to the high incidence of postoperative reflux (19). Intravenous antibiotic prophylaxis should be administered for a standard non-cardiac thoracic procedure and may include cefazolin or clindamycin (16-20).
At the time of surgery, 6–8 mg of intravenous dexamethasone is given to reduce swelling during the procedure. At the start of endoscopy, the starting peak and plateau airway pressures are confirmed with the anesthesiologist. We utilize this baseline along with abdominal examination to understand if capnoperitoneum is potentially compromising the ability to ventilate the patient and requires gastric decompression and/or needle decompression or the peritoneum. Detection of capnothorax and capnoperitoneum can also be carried out intraoperatively by anesthesia using hemodynamic alterations and abdominal exams.
Technique of POEM
POEM is performed with a high definition endoscope that has an associated auxiliary water port and distal attachment of a straight cap. Carbon dioxide insufflation is required over air insufflation because of decreased complications such as bleeding, perforation, and pneumoperitoneum and pneumothorax (21).
The basic technique of POEM involves five major steps as outlined by Inoue, Swanström and Stavropoulos (16,22,23): (I) patient position and planning endoscopy; (II) entry into the submucosal space; (III) creation of a submucosal tunnel; (IV) endoscopic myotomy; (V) closure of the mucosal entrance.
Patient positioning and planning endoscopy
Patients undergoing POEM are placed supine under general endotracheal anaesthesia with the endoscopist standing on the patient’s left at the level of the patient’s head. Access and exposure of the abdomen is needed to facilitate evaluation of capnoperitoneum and if necessary subsequent decompression with a Veress needle.
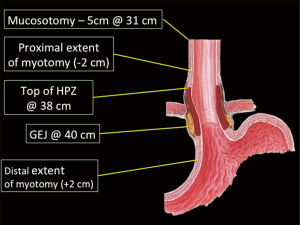
An initial endoscopy is performed to clear any secretions and residual food material from the patient’s esophagus. An over tube is advanced into the esophagus and secured at the teeth. Once in place, the esophagus is evaluated and a measurement taken of the gastroesophageal junction (GEJ) (Figure 1). This distal extent of the myotomy is planned 2 cm distal to the GEJ and the proximal extent of the myotomy marked 3–4 cm proximal to the GEJ to include the high-pressure zone. The mucosal entrance or mucosotomy is marked 5 cm proximal to the start of the myotomy.
Entry into the submucosal space (Figure 2)

To enter the submucosal space, 3–4 mL of normal saline and dilute methylene blue is injected into the submucosal space to raise a “wheal”. The mucosa is incised with an endoscopic knife in a longitudinal orientation for approximately 1 cm. The areolar tissue of the submucosal space is divided until the circular muscle fibers are identified. The clear cap at the end of the endoscope is navigated into the space with additional injections of dilute methylene blue. Once inside the space, the muscle is oriented on the right side and the mucosa on the left when the entry sight is located at the 2–3 o’clock position.
Creation of the submucosal tunnel (Figure 3)

Once inside the submucosal space and oriented, the areolar tissue is divided just along the muscular layer staying away from the mucosal side. Small vessels may be cauterized with the endosurgical knife whereas larger vessels may need to be coagulated with a grasping forceps. The tunnel should be widened by dissecting approximately 1/3 of the circumference of the esophagus. This provides a measure of mobility to maneuver the endoscope. The tunnel is dissected until the planned distal extent is reached. There are several methods to confirm the distal extent has been reached though we favor placing a 5 mm endoscope into the native esophageal lumen into the stomach. In retroflexion, the light of the operative endoscope within the tunnel can be seen and the distal extent of tunneling can be assessed (Figure 4).

Endoscopic myotomy (Figure 5)

Once it has been determined the tunnel is appropriately long, the scope is pulled back to the level of the start of the planned myotomy. Again, using the surgical knife, the circular fibers are divided while preserving the longitudinal fibers. The myotomy is extended distally until the end of the tunnel is reached. Often the GEJ demonstrates muscle fibers in multiple orientations.
Closure of the mucosal entrance (Figure 6)

The mucosal opening is most commonly closed with endoscopic clips from distal to proximal. The first clip is place just past the mucosal opening to create a “ridge” by everting the mucosal edges. This facilitates placement of the next clip and so forth. Alternatively, the mucosal opening can be reapproximated by an endoscopic suture device (Figure 7). We have found this is best accomplished with two figures of 8 sutures rather than a running suture.
Adequacy of the myotomy post POEM
The adequacy of the myotomy post POEM is usually assessed in one of two ways. First, it can be grossly assessed by direct visualization and passage of the gastroscope. Completeness of the myotomy confirmed when the sphincter easily opens with gentle insufflation. Second, a more objective method is to assess the LES before and after myotomy (Figures 8,9) with an endoluminal functional lumen imaging probe catheter (EndoFLIP, Crospon, Galway, Ireland) to assess the completeness of the myotomy (29,30). This device measures the compliance of the tissue it opposes and provides four measurements: compliance, diameter, cross sectional surface area and distensibility. When used intraoperatively before and after myotomy, it can be used to confirm improvements in all parameters after myotomy (31). Unfortunately, threshold levels for the device have not been correlated to clinical outcomes that can reassure the surgical endoscopist that the myotomy is adequate.


Post-operative POEM care
Patients are transferred to the regular surgical floor post procedure on intravenous fluids and nil per os. A water soluble contrast study is obtained on post-operative day one to assess for the presence of intramural and full thickness leakage. If no defects are detected, patients are typically started on a clear liquid diet on post-operative day 1. Clear liquid is maintained for 24–48 hours and then advanced to full liquids for 5 days. A soft to regular diet is begun on post-operative day 6 or 7. Patients are discharged home on a daily proton pump inhibitor.
Patients are seen for routine follow up at 2 and 6 weeks. At the 6 week visit, the proton pump inhibitors are weaned off unless the patient experiences heartburn or indigestion. At 6 months, patients are encouraged to undergo upper endoscopy, pH testing and post POEM manometry to assess for the presence of asymptomatic GERD and assess the completeness of myotomy. At 12 months from POEM, the patients undergo a timed barium swallow and are routinely seen at 2-year intervals with periodic testing with either an upper endoscopy or timed barium swallow based on the presence of symptoms.
Complications
Complications of POEM are generally uncommon though there remains concern for the feared complication of esophageal perforation and mediastinitis. It is thought that preoperative mucosal edema is a common cause of operative mucosal injuries because it makes closure difficult and perforation easier. Edema has been seen in 8% of patients in a retrospective study of over 1,600 patients (32). The inspection step of the procedure should be carried out thoughtfully before proceeding with POEM.
Major adverse events associated with POEM include: mucosal injury, delayed mucosal closure failure, delayed bleeding, hydrothorax, and pneumothorax. In the large retrospective study previously mentioned, delayed mucosal closure failure occurred in 0.8%, delayed bleeding in 0.2%, hydrothorax requiring intervention in 0.5%, pneumothorax requiring intervention in 1.5% (32). A review of multiple outcome reports showed similar percentages and also identified pneumomediastinum, pneumoperitoneum, and subcutaneous emphysema as common post-operative findings (33). Many of these issues are minimized with the use of carbon dioxide insufflation, which allows for quicker dissipation of excess gas (34). Obviously, there is the issue of a learning curve with outcome reports from single centers and as surgeons master the POEM procedure complications should decrease in rate and surveillance of risk factors improves (32,33).
Outcomes
Single arm studies
The treatment of achalasia is a balance between the relief of symptoms particularly dysphagia and the development of complications particularly GERD. POEM has resulted in significant improvements in all measures used to assess the relief of dysphagia and as a result the ability to eat. There have been significant improvements in the Eckardt score with an average reduction of 6.38 from baseline. Accordingly, the reduction in the LES pressures was 22.8 mmHg (Table 1). Several studies also reported objective improvement in esophageal emptying on barium esophagram (38,43,44). However, it should be recognized that the median follow up for most studies remains short and it will be important to follow and understanding whether POEM will be durable in the longer term. The longest reported follow up is around 3 years and shows continued efficacy in relief of dysphagia (35).
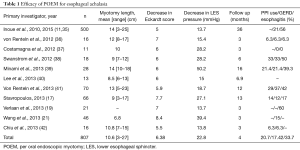
Full table
The development of GERD remains the Achilles heel of achalasia treatment. One of the major concerns as POEM was introduced was the fact that there was no partial fundoplication to provide some evidence of a reflux barrier. It was argued that by leaving the native esophageal hiatus intact and only dividing the inner circular muscle that this might limit the degree of reflux. Most of these initial series (Table 1) reported symptomatic GERD in 0–37% of patients but reported rates of reflux esophagitis can be as high as 65% and thought to be easily controlled with a single dose of PPIs (35). At least one study has highlighted that patients with a hiatal hernia may be at increased risk for erosive esophagitis and GERD post POEM and suggest that this may be a reason to exclude such patients from POEM (44).
Comparison to laparoscopic Heller myotomy (LHM)
In comparison to LHM with or without fundoplication, POEM has demonstrated similar outcomes in relieving dysphagia as evidenced by the similar decreases in Eckardt score and LES pressures when compared to LMH (Table 2). This is not surprising since the myotomy being performed is essentially the same as to what is performed during LHM.
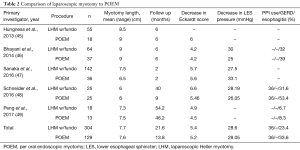
Full table
The development of reflux and PPI use does not appear to be significantly different between LHM and POEM (46,48). However, these series have small numbers and these outcomes may change when larger studies are conducted. A recent meta-analysis concluded that there was a trend toward a significant reduction in the development of symptomatic GERD with LHM (50). The larger concern are that many patients do not perceive reflux symptoms yet have positive objective pH scores and reflux esophagitis. Because of this, we believe it is imperative to evaluate all patients post myotomy with pH testing to confirm a diagnosis of GERD (48).
POEM after LHM and other procedures
Each of the alternative therapies for achalasia—Botox injection, pneumatic dilation and LHM—involve or access the submucosal space and potentially could limit the ability to use POEM in these settings. Several retrospective studies of patients undergoing POEM after various previous interventions found that POEM was feasible in most patients and did not result in worse outcomes (51-54). However, when grouped into three categories based on the intervention, dilation of the esophagus and presence of a sigmoid shaped esophagus, injections and small caliber dilations have little impact on outcomes, but those patients that underwent forceful pneumatic dilation and/or a prior myotomy required almost double the operative time (52).
Conclusions
POEM is a minimally invasive, natural orifice procedure that has undergone rapid adoption across the world for the treatment of achalasia. It has been shown to be relatively safe with limited complications in general and rarely life threatening issues. The procedure has been shown to relieve the symptoms of dysphagia, restore the ability to eat but can result in the development of GERD at similar but slightly higher rates than LHM with partial fundoplication. Longer term data is required to confirm its place in the management of achalasia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Willis T. Pharmaceutice rationalis sive diatribe de medicamentorum operationibus in humano corpore. London: Hagae-comitis, A. Leers, 1674.
- Heller E. Extramucöse Cardioplastie beim chronischen Cardiospasmus mit Dilatation des Oesophagus. Mitt Grengeb Med Chir 1913;2:141-9.
- Ellis FH Jr, Crozier RE, Watkins E Jr. Operation for esophageal achalasia. Results of esophagomyotomy without an antireflux operation. J Thorac Cardiovasc Surg 1984;88:344-51. [PubMed]
- Pellegrini CA, Leichter R, Patti M, et al. Thoracoscopic esophageal myotomy in the treatment of achalasia. Ann Thorac Surg 1993;56:680-2. [Crossref] [PubMed]
- Spechler SJ. Pneumatic dilation and laparoscopic Heller's myotomy equally effective for achalasia. N Engl J Med 2011;364:1868-70. [Crossref] [PubMed]
- Heller E. Extramukose cardiaplastik bein chronischen cardiospasmus ut dilatation des esophagus. Mitt Grenz Med Chir 1914;27:141-9.
- Ellis FH Jr, Kiser JC, Schlegel JF, et al. Esophagomyotomy for esophageal achalasia: experimental, clinical, and manometric aspects. Ann Surg 1967;166:640-56. [Crossref] [PubMed]
- Pellegrini C, Wetter LA, Patti M, et al. Thoracoscopic esophagomyotomy. Initial experience with a new approach for the treatment of achalasia. Ann Surg 1992;216:291-6. [Crossref] [PubMed]
- Patti MG, Pellegrini CA, Horgan S, et al. Minimally invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg 1999;230:587-93. [Crossref] [PubMed]
- Stewart KC, Finley RJ, Clifton JC, et al. Thoracoscopic versus laparoscopic modified Heller Myotomy for achalasia: efficacy and safety in 87 patients. J Am Coll Surg 1999;189:164-9; discussion 169-70. [Crossref] [PubMed]
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- Ortega JA, Madureri V, Perez L. Endoscopic myotomy in the treatment of achalasia. Gastrointest Endosc 1980;26:8-10. [Crossref] [PubMed]
- Pasricha PJ, Hawari R, Ahmed I, et al. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy 2007;39:761-4. [Crossref] [PubMed]
- Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc 2007;65:688-94. [Crossref] [PubMed]
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- Grimes KL, Inoue H. Per Oral Endoscopic Myotomy for Achalasia: A Detailed Description of the Technique and Review of the Literature. Thorac Surg Clin 2016;26:147-62. [Crossref] [PubMed]
- Stavropoulos SN, Modayil RJ, Friedel D, et al. The International Per Oral Endoscopic Myotomy Survey (IPOEMS): a snapshot of the global POEM experience. Surg Endosc 2013;27:3322-38. [Crossref] [PubMed]
- Inoue H, Sato H, Ikeda H, et al. Per-Oral Endoscopic Myotomy: A Series of 500 Patients. J Am Coll Surg 2015;221:256-64. [Crossref] [PubMed]
- Verlaan T, Rohof WO, Bredenoord AJ, et al. Effect of peroral endoscopic myotomy on esophagogastric junction physiology in patients with achalasia. Gastrointest Endosc 2013;78:39-44. [Crossref] [PubMed]
- Stavropoulos SN, Desilets DJ, Fuchs KH, et al. Per-oral endoscopic myotomy white paper summary. Surg Endosc 2014;28:2005-19. [Crossref] [PubMed]
- Wang J, Tan N, Xiao Y, et al. Safety and efficacy of the modified peroral endoscopic myotomy with shorter myotomy for achalasia patients: a prospective study. Dis Esophagus 2015;28:720-7. [Crossref] [PubMed]
- Swanström LL, Rieder E, Dunst CM. A stepwise approach and early clinical experience in peroral endoscopic myotomy for the treatment of achalasia and esophageal motility disorders. J Am Coll Surg 2011;213:751-6. [Crossref] [PubMed]
- Stavropoulos SN, Harris MD, Hida S, et al. Endoscopic submucosal myotomy for the treatment of achalasia (with video). Gastrointest Endosc 2010;72:1309-11. [Crossref] [PubMed]
- Smith SP, Louie BE. Entry into the submucosal space. Asvide 2017;4:369. Available online: http://www.asvide.com/articles/1683
- Smith SP, Louie BE. Creation of the submucosal tunnel. Asvide 2017;4:370. Available online: http://www.asvide.com/articles/1684
- Smith SP, Louie BE. Placing a 5 mm endoscope into the native esophageal lumen into the stomach. Asvide 2017;4:371. Available online: http://www.asvide.com/articles/1685
- Smith SP, Louie BE. Endoscopic myotomy. Asvide 2017;4:372. Available online: http://www.asvide.com/articles/1686
- Smith SP, Louie BE. Closure of the mucosal entrance. Asvide 2017;4:373. Available online: http://www.asvide.com/articles/1687
- Teitelbaum EN, Soper NJ, Pandolfino JE, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc 2015;29:522-8. [Crossref] [PubMed]
- Friedel D, Modayil R, Stavropoulos SN. Per-oral endoscopic myotomy: major advance in achalasia treatment and in endoscopic surgery. World J Gastroenterol 2014;20:17746-55. [PubMed]
- Familiari P, Gigante G, Marchese M, et al. EndoFLIP system for the intraoperative evaluation of peroral endoscopic myotomy. United European Gastroenterol J 2014;2:77-83. [Crossref] [PubMed]
- Zhang XC, Li QL, Xu MD, et al. Major perioperative adverse events of peroral endoscopic myotomy: a systematic 5-year analysis. Endoscopy 2016;48:967-78. [Crossref] [PubMed]
- Crespin OM, Liu LWC, Parmar A, et al. Safety and efficacy of POEM for treatment of achalasia: a systematic review of the literature. Surg Endosc 2017;31:2187-201. [Crossref] [PubMed]
- Ren Z, Zhong Y, Zhou P, et al. Perioperative management and treatment for complications during and after peroral endoscopic myotomy (POEM) for esophageal achalasia (EA) (data from 119 cases). Surg Endosc 2012;26:3267-72. [Crossref] [PubMed]
- Inoue H, Sato H, Ikeda H, et al. Per-Oral Endoscopic Myotomy: A Series of 500 Patients. J Am Coll Surg 2015;221:256-64. [Crossref] [PubMed]
- von Renteln D, Inoue H, Minami H, et al. Peroral endoscopic myotomy for the treatment of achalasia: a prospective single center study. Am J Gastroenterol 2012;107:411-7. [Crossref] [PubMed]
- Costamagna G, Marchese M, Familiari P, et al. Peroral endoscopic myotomy (POEM) for oesophageal achalasia: preliminary results in humans. Dig Liver Dis 2012;44:827-32. [Crossref] [PubMed]
- Swanstrom LL, Kurian A, Dunst CM, et al. Long-term outcomes of an endoscopic myotomy for achalasia: the POEM procedure. Ann Surg 2012;256:659-67. [Crossref] [PubMed]
- Minami H, Isomoto H, Yamaguchi N, et al. Peroral endoscopic myotomy for esophageal achalasia: clinical impact of 28 cases. Dig Endosc 2014;26:43-51. [Crossref] [PubMed]
- Lee BH, Shim KY, Hong SJ, et al. Peroral endoscopic myotomy for treatment of achalasia: initial results of a korean study. Clin Endosc 2013;46:161-7. [Crossref] [PubMed]
- Von Renteln D, Fuchs KH, Fockens P, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology 2013;145:309-11. [Crossref] [PubMed]
- Chiu PW, Wu JC, Teoh AY, et al. Peroral endoscopic myotomy for treatment of achalasia: from bench to bedside (with video). Gastrointest Endosc 2013;77:29-38. [Crossref] [PubMed]
- Teitelbaum EN, Soper NJ, Santos BF, et al. Symptomatic and physiologic outcomes one year after peroral esophageal myotomy (POEM) for treatment of achalasia. Surg Endosc 2014;28:3359-65. [Crossref] [PubMed]
- Worrell SG, Alicuben ET, Boys J, et al. Peroral Endoscopic Myotomy for Achalasia in a Thoracic Surgical Practice. Ann Thorac Surg 2016;101:218-24. [Crossref] [PubMed]
- Hungness ES, Teitelbaum EN, Santos BF, et al. Comparison of perioperative outcomes between peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy. J Gastrointest Surg 2013;17:228-35. [Crossref] [PubMed]
- Bhayani NH, Kurian AA, Dunst CM, et al. A comparative study on comprehensive, objective outcomes of laparoscopic Heller myotomy with per-oral endoscopic myotomy (POEM) for achalasia. Ann Surg 2014;259:1098-103. [Crossref] [PubMed]
- Sanaka MR, Hayat U, Thota PN, et al. Efficacy of peroral endoscopic myotomy vs other achalasia treatments in improving esophageal function. World J Gastroenterol 2016;22:4918-25. [Crossref] [PubMed]
- Schneider AM, Louie BE, Warren HF, et al. A Matched Comparison of Per Oral Endoscopic Myotomy to Laparoscopic Heller Myotomy in the Treatment of Achalasia. J Gastrointest Surg 2016;20:1789-96. [Crossref] [PubMed]
- Peng L, Tian S, Du C, et al. Outcome of Peroral Endoscopic Myotomy (POEM) for Treating Achalasia Compared With Laparoscopic Heller Myotomy (LHM). Surg Laparosc Endosc Percutan Tech 2017;27:60-4. [PubMed]
- Marano L, Pallabazzer G, Solito B, et al. Surgery or Peroral Esophageal Myotomy for Achalasia: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2016;95:e3001. [Crossref] [PubMed]
- Sharata A, Kurian AA, Dunst CM, et al. Peroral endoscopic myotomy (POEM) is safe and effective in the setting of prior endoscopic intervention. J Gastrointest Surg 2013;17:1188-92. [Crossref] [PubMed]
- Louie BE, Schneider AM, Schembre DB, et al. Impact of prior interventions on outcomes during per oral endoscopic myotomy. Surg Endosc 2017;31:1841-8. [Crossref] [PubMed]
- Orenstein SB, Raigani S, Wu YV, et al. Peroral endoscopic myotomy (POEM) leads to similar results in patients with and without prior endoscopic or surgical therapy. Surg Endosc 2015;29:1064-70. [Crossref] [PubMed]
- Jones EL, Meara MP, Pittman MR, et al. Prior treatment does not influence the performance or early outcome of per-oral endoscopic myotomy for achalasia. Surg Endosc 2016;30:1282-6. [Crossref] [PubMed]
Cite this article as: Smith SP, Louie BE. The current state of per oral endoscopic myotomy for achalasia. J Vis Surg 2017;3:122.


