The results of video-assisted thoracoscopic thymectomies in Saint Petersburg, Russia: 20-year of experience
Introduction
Today, advantages of minimally invasive surgery, particularly video-assisted thoracoscopic thymectomy (VATS-TE) are well established. However, there is no consensus what VATS-TE strategy is optimal for treatment of autoimmune myasthenia gravis (MG) and thymoma.
In this report, we show the results of 254 VATS-TE and suggest that VATS-TE combined with an additional cervical approach does not give any advantages compared to VATS-TE alone, which is equally effective, but reduces the risks of specific postoperative complications. Here, we summarize our 20-year experience in performing VATS-TE, and demonstrate their effectiveness and safety in complex treatment of MG and thymomas both in a short and in a long term.
Methods
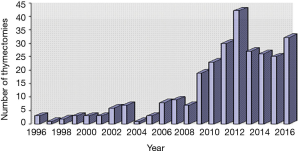
This study was approved by the local Ethical Committee which complied with the Helsinki Declaration [2013]. All patients gave their written consent to participate in the study. Two hundred eighty-one patients were examined and underwent surgery in the Saint Petersburg City Hospital #2 and in the Sokolov’s Clinical Hospital #122 during the period between 1996 and 2016. Among the patients, 82 were males and 199 were females aged from 14 to 87 years, with the mean age 41.9±17.0 years (48.3±16.4 in men and 39.4±16.6 in women) (Figure 1).
In most cases, 90% (254 out of 281 patients) underwent VATS-TE: in 62% (175 patients), VATS-TE alone (T-2a according to classification of MGFA (1,2), and in 28% (79 patients), VATS-TE with additional cervical approach according to the technique described by Novellino et al. (3). In almost all of the cases (92%), we used right-side approach. Left-side approach was used only in cases of a considerable thymic prevalence at the left. Surgeries were carried out using three thoracic ports. Open access surgeries were performed only in 10% of all thymectomies; of them sternotomies (T-3v) were performed in 14 patients, and thoracotomies in 13 patients. Open access was used for cases of thymomas larger than >8 cm. In 6 cases, VATS-TE was converted into an opened surgery; of those, 4 to the thoracotomy, and 2 to sternotomy). The reasons for conversions were large tumors, invasion of pericardium, phrenic nerve, lung or brachiocephalic vein.
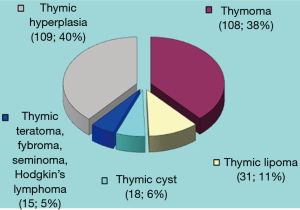
Thymic morphology revealed thymomas in 38% (108 patients) and thymic hyperplasia in 40% (109 patients). Thymolipoma was found in 11% (31 patients), thymic cysts and tumors of extrathymic origin (seminoma, fibroma, teratoma and Hodgkin lymphoma) were diagnosed in 11% (33 patients) (Figure 2). In most cases—64% (179 patients), thymic pathology was associated with MG. The combination of MG and a thymoma (thymoma-associated MG) was revealed at 90 patients (32% of all patients or 50% of all MG patients).
For definition of the functional class of MG we used the MGFA clinical classification (1). The majority of the patients belonged to the class II (75 patients/42%) and class III (65 patients/36%).
To evaluate the degree of tumor progression we used the classification of thymomas by Masaoka et al., which is based on the capsule invasion (4). Thymoma of class I was revealed in 44 patients (41%), class II in 55 (51%), and class III in 9 (8%).
Patient selection
Preoperative evaluation of the patients included: the analysis of clinical data, assessment of the neurologic status, electroneuromyography (ENMG), proserin (Tensilon) test, computer tomography (CT) of the thorax, in some cases magnetic resonance imaging (MRI), ultrasound of the thyroid gland, T3, T4 and TTG; respiratory tests (spirography or body plethysmography), and serological determination of autoantibodies to skeletal muscle (SM), and/or to acetylcholine receptors (AHR).
The major indications for VATS-TE were thymic tumors without signs of invasion into the surrounding tissue, <8 cm in size, and/or generalized MG of functional class II–IV in patients older that 16 years, resistant to conservative therapy, as well as intolerance of such therapy.
Exclusion criteria were advanced stages of somatic diseases, myasthenic crisis with acute bulbar symptoms, intolerance to one-lung ventilation, invasive thymoma (Masaoka class III) or tumors >8 cm.
VATS-TE were perform under general anaesthesia and single-lung ventilation with double-lumen tube (Figure 3).

The aim of an extended VATS-TE a radical removal of all thymic tissue. For that, all the visible thymic tissue was removed en block without damaging the capsule, together with perithymic and pericardiac adipose tissue of the anterior mediastinum, aortic window and infra thyroid tissue of the neck, which might contain “aberrant” thymic lobes. A chest tube was inserted through the lower port. Usually, the chest tube was removed on day 1 or 2 if no signs of air-leak and fluid drainage not exceeding 100–150 mL per day. The patients were observed up to 6–7 days.
Assessment of MGFA postintervention status
Long term results of surgical treatment of patients with MG were followed up for 1–15.5 years after the surgery. We used MGFA postinterventional status criteria, modified so that pharmacologic remission (PR), minimal manifestations (MM) and improved (I) definitions were combined in “improvement”. Thus, we divided the patients into five groups depending on the outcome of thymectomy: (I) a complete and stable remission (lack of clinical manifestations within at least one year without medications) [Complete Stable Remission on the MGFA 2000 scale (1)]; (II) improvement—alleviation of symptoms with a continued use of medicines, or a reduction in drug dose compared to the preoperative, or a decrease in MGFA score (corresponds to the PR, MM on the MGFA scale); (III) stabilization—lack of effect, no improvement in symptoms or doses of medicines (corresponds Unchanged on the MGFA scale); (IV) worsening of symptoms or increase in the dose of medicines (worse on the MGFA scale); (V) lethal outcome from AM or complications of therapy of AM or within 30 days after the surgery (died of MG on the MGFA scale). To test the efficacy of surgical treatment, we compared changes of the mean MGFA values before and after the surgery with MGFA changes during a 3-year period in MG patients that did not undergo surgery.
For compare changes in the quality of life in MG patients VATS-TE we used a questionnaire of MG QOL-15 (6-8), specific for MG.
Statistical analysis
Data were analyzed using GraphPad Prizm V software (GraphPad Software, Inc., La Jolla, CA, USA). Paired Mann-Whitney test was used for comparison of values before and after the surgery, with P<0.05 considered significant.
Results
We compared VATS-TE alone with VATS-TE plus cervical approach in terms of surgery duration time, blood loss during the surgery and after it, time of the chest tube removal, and the incidence of early postoperative complications. As show in Table 1, there was no significant difference between these two strategies, and VATS-TE plus cervical dissection does not provide any advantage over VATS-TE alone. Instead, there is a trend towards reduction of surgery duration time, blood loss and postoperative complications in the VATS-TE alone group.
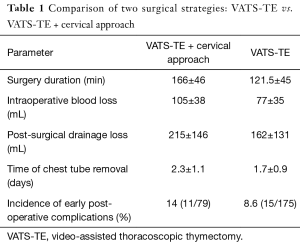
Full table
Early postoperative complications
Table 2 shows that early postoperative complications developed in 26 patients (10%). In 14 of them (5.5%) exacerbation of MG symptoms occurred, and 9 of these developed myasthenic crisis and required pulmonary ventilation with duration from 9h to 30 days. One patient developed a minor hemothorax which resolved after an additional drainage of the pleural cavity. A clotted hemothorax in another patient required a repeated surgery—re-VATS on the 3rd day, hemostasis and blood clot removal. Pleural complications developed in 4 patients: pneumothorax [1], a prolonged air leakage [1], pleuritis [1], and chylothorax [1]. Among infectious complications, we observed suppuration of a drainage aperture [1], and a postoperative pneumonia [1]. Other complications were represented by a subcutaneous emphysema [1], atrial fibrillation [1], paresis of the right dome of the diaphragm [1], and paresis of a voice cord due to a damage to the nervus laryngeus recurrens (3 patients).

Full table
Two hundred fifty three out of 254 patients were discharged from the hospital; one patient died due to decompensation of MG and complication linked to a prolonged pulmonary ventilation. Thus, the postoperative hospital mortality was 0.4%. Patients with thymomas were followed up from 1 to 17 years after the surgery. The local recurrence of the thymoma developed in one female patient 7 years after a combination treatment.
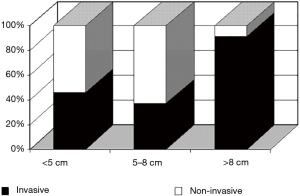
We analyzed the association between thymoma size and invasiveness and found that with an increase in tumor size the proportion of invasive tumors increases (Figure 4) (9). Tumors larger than 8 cm consisted mostly of invasive ones (91%; P<0.01; Fisher’s exact test). This finding suggests that, if there are no macroscopic signs of invasive growth, 8 cm may be considered the threshold in selecting the surgical strategy for thymectomy.
Long term results of VATS-TE in MG patients
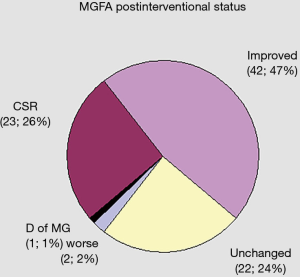
Long term results of surgical treatment for MG could be studied in 92 patients which were followed up for up to 15.5 years after thymectomy. The mean follow-up duration was 66 months, and at this time point, complete remission and improvement rates were 26% (23 patients) and of 47% (42 patients), respectively (Figure 5). In 22 patients (24%), the disease stabilized, and in 2 patients (2%), symptoms became worse. One patient decided to stop taking prescription medicines, developed myasthenic crisis and died, whereas three others died of reasons irrelevant to MG, thymoma or the surgery.
Effect of surgical approach on long term outcomes in MG patients
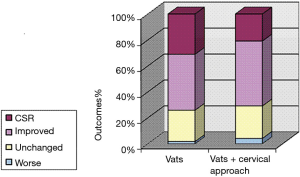
We have compared long term outcomes in 59 patients that underwent VATS-TE to those in 24 patients that underwent VATS-TE plus cervical approach. As shown in Figure 6, there was no significant difference in the proportion or outcomes.
Quality of life
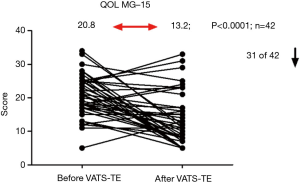
Quality of life was assessed in 42 MG patients before and after VATS-TE using a specialized questionnaire (MG-QOL15) (Figure 7). The results of this survey are expressed in such a way that a lower score indicates a higher quality of life. As shown in Figure 7, the majority of the patients (31 out of 42, or 74%) improved their quality of life in a long term: the mean score decreased from 20.8 to 13.2 (P<0.0001). Therefore, the majority of MG patients subjectively noted a significant improvement of their quality of life after the surgery. Although, some patients noted certain periodic “unpleasant feelings” in the area of surgery within the first 6 months after the operation, those were gone later, spontaneously. All the patients were completely satisfied by the cosmetic results of the surgery.
Clinical improvement time
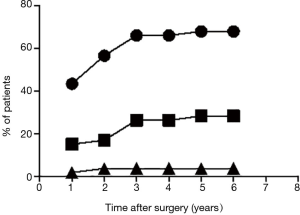
We analyzed the time when, either the patients, or neurologists (using MGFA score) noted the improvement of clinical manifestations of MG after VATS-TE. The number of patients that improved their symptoms had been accumulated during the first 3 years, after which time there was no additional increase (Figure 8). Cumulative increase of a proportion of persons with clinical improvement is presented in Figure 8.
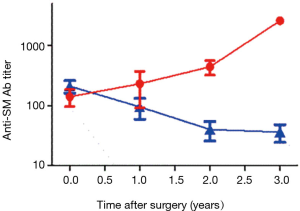
Anti-SM antibody titers and clinical improvement time
We analyzed changes in the anti-SM autoantibody titers in 40 patients during a 3-year follow up after VATS-TE. As show in Figure 9, there was a progressive decline of anti-SM autoantibody concentration throughout this period in patients that improved their clinical course of MG, and this was not observed in patient, who did not improve. Clinical improvement, in general, coincided with the reduction of anti-SM antibody titers. Therefore, the final outcome of the surgery should be fully appreciated not earlier than 3 years after the operation.
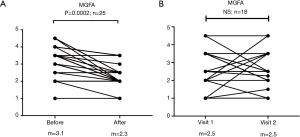
Comparison of surgical and conservative treatments
When comparing mean MGFA score before and after the treatment, we found that, in most of the patients, after complex (including surgical) treatment, a notable decrease in MG severity was observed by 1 point (from 3.5 before the surgery, to 2.5 after the surgery; P=0.0002) (Figure 10A). There was no change in the MGFA score in the group of MG patients that did not undergo surgery during a comparable period of time (Figure 10B). This data strongly argues in favor of effectiveness of VATS-TE in alleviation of MG severity.
Discussion
Nowadays, advantages of minimally invasive surgery, specifically VATS-TE raise no doubts: minimal injury, low intraoperative blood loss, reduced pain and the need for pain killers including narcotic analgesics, rapid recovery and short hospital stay time, early return to habitual life and work, and a much better cosmetic effect have all been well documented. With that in mind, patients agree to the surgical treatment easier, earlier after the onset of the disease, which further contributes to beneficial outcomes (10-16). However, there is still no consensus which strategy for thymectomy to prefer in cases of MG and/or thymoma.
We started to perform thymectomies 20 years ago. Since then, our view on the techniques, strategies, and preferences underwent certain evolution. Speaking of thymomas, we changed our mind on their critical size. Some surgeons insisted that radical removal of a thymoma is possible only with an open access, whereas VATS-TE does not provide oncological “reliability” (10). Others considered that small non-invasive thymomas can be removed by VATS (17). Our data indicate that the size threshold, after which thymomas should not be removed by VATS, is 8 cm, since a proportion of invasive tumors among those under that size is low (Figure 4). For tumors larger than 8 cm, an open approach should be selected.
The surgical volume is determined by the necessity for a complete removal of thymic tissue and surrounding fat regardless tumor morphology and the selected surgical access. Using VATS-TE, we excised noninvasive thymomas up to 8 cm. Long term postoperative observation (17 years) revealed recurrence of the tumor only in one patient (0.9%).
Our data suggest that VATS-TE are rather radical and are not associated with a higher frequency of a recurrence of thymomas if compared to open surgeries (Figure 6).
In 64% of our patients, thymus pathology was associated with MG. While performing VATS-TE in these patients, we complied to the statement of A. Jaretzki III, that is to removal of all thymic tissue, including aberrant mediastinal and top pole segments (18,19). We believed that the technique suggested by Novellino in 1994—VATS-TE plus cervical approach—satisfies these requirements (3). To reduce the duration of surgery, we carried it out by 2 teams of surgeons—on the neck, and on the thorax. However, we faced a number of complications due to the cervical access: in 3 patients, there was a temporary paresis of the voice cord due to the damage to nervus laryngeus recurrens; and, in one patient, a chylothorax due to the damage of the thoracic lymph duct.
Meanwhile, our experience increased, which allowed to reduce the surgery duration time. The availability of ultrasonic knife and modern hemostatic materials allowed to further shorten the operation time, to reduce the volume of intraoperative blood loss, and improvement in visualization made it possible to access cervical thymic lobes from the thorax.
The analysis of long term results of thymectomies in MG patients showed that VATS-TE alone is not inferior to VATS-TE plus cervical approach (Figure 6). Since VATS-TE plus cervical access does not give any advantages surgery duration and completeness of tissue removal, but, on the contrary, increases injury and is prone to specific complications, use this combined approach only when most of the thymic tissue is located above the brachiocephalic vein, or when there is a thymic tissue regrowth in the neck area.
According to the literature, thymectomy is effective in 80% of GM patients (20). A stable remission occurs in 19–50% of cases (21-23) after open access surgeries; after VATS-TE in 30–36% of patients (12,23-25), and according to Tomulescu et al. (15), up to 61%; clinical improvement and/or a reduction of the dose of medicines is observed in 30–50% of patients. We report permanent remission in 26% of patients, and clinical improvement in 47%. Only 2% of patients had worsening of clinical symptoms, and in 1% there was a lethal outcome (Figure 5). Thus, VATS-TE can be considered a safe and an efficient method for treatment of MG.
There is an opinion that VATS significantly limits visualization of important mediastinal structures, and a complete resection of the thymus is impossible (26). However, a modern equipment and the use of oblique optics provide a good visualization of all areas of the anterior mediastinum and the cervical area, which allows for a complete removal of the thymus and the parathymic adipose tissue (10,27-29). Contrary to the suggestions by Mineo and Pompeo (10), we are confident that the access to the top areas of cervical lobes does not require an additional cervical dissection, since this tissue completely reachable from the mediastinum.
According to MGFA, MG treatment is considered successful after achievement of a complete permanent remission (1). A retrospective analysis by Buckingham et al. showed that the incidence of permanent remissions in MG patients after conservative therapy is only 8%, and mortality is as high as 43%, while after thymectomy the values are 34% and 14%, respectively (30). Gronseth and Barohn (31) analyzed results of works from 1966 to 2000 and came to a conclusion that the frequency of improvements and stable remissions in MG patients with after thymectomy is 1.5–2 times higher, than in the patients receiving only conservative therapy. Similar data were obtained by Luo et al. (32), who analyzed publications from 1980 to 2013. Our results are in agreement with this data, and we show that a complex treatment of MG which includes surgery is more efficient, than conservative therapy alone (Figure 10).
The combination of immunosuppressive therapy with glucocorticoids, is an efficient method of treatment of MG, but leads to the development of a large number of side effects on cardiovascular system, kidneys, is associated with insulin resistance and type 2 diabetes, etc. (33,34). Surgical treatment allows to reduce the need for immunosuppressive drugs or to make them completely unnecessary. Therefore VATS-TE is a method of the choice in a complex treatment of MG.
Clinical results readout time varies among the authors. Some of them suggest to judge about the effect in a year after the surgery (35). Others allow waiting 3, 6, 9 and even 15 years (26,28,36-38). In our previous report (9), we have shown that only in 5% of cases remission of MG was observed within the first year after the surgery, within the second year in 46%, and only after the third year the proportion of postoperative remissions or improvements exceeded 87%. Cumulative incidence of remissions and improvements presented in Figure 8 are in agreement with our earlier data. Improvements remarkably coincided in time with lowering the levels o anti-SM autoantibodies (Figure 9). Thus, it is important to estimate the clinical outcome of thymectomy on the MG severity not earlier than 3 years after the surgery.
Conclusions
- The extended VATS-TE is a radical, efficient, safe, technically feasible and a well-tolerated surgery. It improves the course of MG as a part of multimodality treatment more efficiently than a conservative therapy alone.
- The course of MG after VATS-TE shows that the cumulative incidence of remissions/improvements reaches its maximum by the 3rd year after the surgery.
- VATS-TE is radical and safe for removal of noninvasive thymomas up to 8 cm in size.
- Additional neck incision (VATS-TE + cervical approach) does not provide further advantages, but rather may be a cause of specific postoperative complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the local Ethical Committee which complied with the Helsinki Declaration [2013]. All patients gave their written consent to participate in the study.
References
- Jaretzki A III, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standarts. Task force of the medical scientific advisory board of the Myasthenia Gravis Foundation of America. Ann Thorac Surg 2000;70:327-34. [Crossref] [PubMed]
- Sonett JR, Jaretzki A III. Thymectomy for Nonthymomatous Myasthenia Gravis - a critical analysis. Ann NY Acad Sci 2008;1132:315-28. [Crossref] [PubMed]
- Novellino L, Longoni M, Spinelli L, et al. Extended thymectomy without sternotomy performed by cervicotomy and thoracoscopic technique in the treatment of myasthenia gravis. Int Surg 1994;79:378-81. [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Yablonsky P, Pischik V, Tovbina MG, et al. VATS thymectomy: right side approach. Asvide 2017;4:325. Available online: http://www.asvide.com/articles/1637
- Mullins LL, Carpentier MY, Paul RH, et al. A disease-Specific measure of quality of life for myasthenia gravis. Muscle Nerve 2008;38:947-56. [Crossref] [PubMed]
- Burns TM, Conaway MR, Cutter GR, et al. Less is more or almost as much: a 15-item quality-of-life instrument for myasthenia gravis. Muscle Nerve 2008;38:957-63. [Crossref] [PubMed]
- Burns TM, Grouse CK, Cutter G, et al. The MG-QOL15 for following the health-related quality of life of patients with myasthenia gravis. Muscle Nerve 2011;43:14-8. [Crossref] [PubMed]
- Pischik VG. Mediastinal neoplasms: principles of differential diagnosis and surgical treatment. Thesis for a DMSci degree. Saint Petersburg, Russia, 2008.
- Mineo TC, Pompeo E. Extended Videothoracoscopic thymectomy in nonthymomatous myasthenia gravis. Thorac Surg Clin 2010;20:253-63. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Massa R, et al. Long-term outcome of thoracoscopic extended thymectomy for nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2009;36:164-9. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Lerut T, et al. Thoracoscopic thymectomy in autoimmune myasthenia: results of left-sided approach. Ann Thorac Surg 2000;69:1537-41. [Crossref] [PubMed]
- Liu CW, Luo M, Mei JD, et al. Perioperative and long-term outcome of thymectomy for myasthenia gravis: comparison of surgical approaches and prognostic analysis. Chin Med J 2013;126:34-40. [PubMed]
- Youssef SJ, Louie BE, Farivar AS, et al. Comparison of open and minimally invasive thymectomies at a single institution. Am J Surg 2010;199:589-93. [Crossref] [PubMed]
- Tomulescu V, Sgarbura O, Stanescu C, et al. Ten-year results of thoracoscopic unilateral extended thymectomy performed in nonthymomatous myasthenia gravis. Ann Surg 2011;254:761-5; discussion 765-6. [Crossref] [PubMed]
- Lashkarizadeh MR, Ajami R, Vahedian M, et al. Video-assisted Thoracoscopic thymectomy as an optimal treatment in myasthenia gravis. Minim Invasive Surg Sci 2013;2:144-8.
- Sakamaki Y, Kido T, Yasukawa M. Alternative choices of total and partial thymectomy in video-assisted resection of noninvasive thymomas. Surg Endosc 2008;22:1272-7. [Crossref] [PubMed]
- Jaretzki A III. Thymectomy for myasthenia gravis: an analisis of the controversies regarding technique and results. Neurology 1997;48:S52-63. [Crossref]
- Jaretzki A 3rd, Wolff M. “Maximal” thymectomy for myasthenia gravis. Surgical anatomy and operative technique. J Thorac Cardiovasc Surg 1988;96:711-6. [PubMed]
- Turner C. A review of myasthenia gravis: pathogenesis, clinical features and treatment. Curr Anaesth Crit Care 2007;18:15-23. [Crossref]
- Jaretzki A, Penn AS, Younger DS, et al. “Maximal” thymectomy for myasthenia gravis. Results. J Thorac Cardiovasc Surg 1988;95:747-57. [PubMed]
- Kaufman A, Palatt J. Is surgical resection justified for myasthenia gravis? Long-term results in over 1000 cases. Semin Thoracic Surg 2016;28:569-71.
- Lin TS, Tsao C, Lee SC, et al. Comparison between video-assisted thoracoscopic thymectomy and transternal thymectomy for myasthenia gravis (analis of 82 cases). Int Surg 2005;90:36-41. [PubMed]
- Yim AP, Kay RL, Ho JKS. Video-assisted thoracoscopic thymectomy for myasthenia gravis. Chest 1995;108:1440-3. [Crossref] [PubMed]
- Manlulu A, Lee TW, Wan I, et al. Video-assisted thoracic surgery thymectomy for nonthymomatous myasthenia gravis. Chest 2005;128:3454-60. [Crossref] [PubMed]
- Kaiser LR. Thoracoscopic resection of the mediastinal tumors and the thymus. Chest Surg Clin N Am 1996;6:41-52. [PubMed]
- Stern LE, Nussbaum MS, Quinlan JG, et al. Long term evaluation of extended thymectomy with anterior mediastinal dissection for myasthenia gravis. Surgery 2001;130:774-8; discussion 778-80. [Crossref] [PubMed]
- Savcenko M, Wendt GK, Prince SL, et al. Video-assisted thymectomy for myasthenia gravis: an update of a single institution experience. Eur J Cardiothorac Surg 2002;22:978-83. [Crossref] [PubMed]
- Rückert JC, Ismail M, Badakhshi H, et al. Thymectomy in myasthenia and/or thymoma. Zentralbl Chir 2014;139:121-32. [Crossref] [PubMed]
- Buckingham JM, Howard FM, Bernatz PE. The value of thymectomy in myasthenia gravis: a computer-assisted matched study. Ann Surg 1976;184:453-8. [Crossref] [PubMed]
- Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review). Neurology 2000;55:7-15. [Crossref] [PubMed]
- Luo Y, Pan D, Chen F, et al. Effectiveness of thymectomy in non-thymomatous myasthenia gravis: a systematic review. J Huazhong Univ Sci Technolog Med Sci 2014;34:942-9. [Crossref] [PubMed]
- Kuzin MI, Gecht BM. Myasthenia. Moscow Medicine 1996.224.
- Gecht BM. Treatment of Myasthenia. Neurological J 2005;5:4-9.
- Blalock A, Mason MF, Morgan HJ, et al. Myasthenia gravis and tumors of the thymic region. Ann Surg 1939;110:544-61. [Crossref] [PubMed]
- Bulkley GB, Bass KN, Stephenson GR, et al. Extended cervicomediastinal thymectomy in the integrated management of myasthenia gravis. Ann Surg 1997;226:324-34; discussion 334-5. [Crossref] [PubMed]
- Oosterhuis HJ. Observation of the natural history of myasthenia gravis and the effect of thymectomy. Ann NY Acad Sci 1981;377:678-90. [Crossref] [PubMed]
- Mantegazza R, Baggi F, Bernasconi P, et al. Video-assisted thoracoscopic extended thymectomy and extended transsternal thymectomy (T-3b) in nonthymomatous myasthenia gravis patients: remission after 6 years of follow up. J Neurol Sci 2003;212:31-6. [Crossref] [PubMed]
Cite this article as: Yablonsky P, Pischik V, Tovbina MG, Atiukov M. The results of video-assisted thoracoscopic thymectomies in Saint Petersburg, Russia: 20-year of experience. J Vis Surg 2017;3:113.