Definitions and standard indications of minimally-invasive techniques in thymic surgery
Introduction
In 2011 the group of thoracic surgeons especially interested in minimally invasive surgery of the thymus was appointed by the International Thymic Malignancy Interest Group (ITMIG) to prepare the recommendations regarding Standard Terms, Definitions, and Policies for Minimally Invasive Resection of Thymoma (1). The aim of the recommendations was to encompass all aspects of the minimally surgery of thymomas, with special attention to differentiate this kind of procedures from open surgery and to define clearly the conditions of high quality the minimally invasive procedures must fulfill. It was not surprising, that such recommendations were rather general, without analyzing specific details. It seems justifiable, therefore, to analyze some aspects of thymic surgery in more detailed way.
The aim of the current paper is not to repeat or change the principles of the minimally invasive surgery of thymomas, which were very clearly presented in the previous article.
Instead of this, it seems important to specify modern indications for minimally invasive procedures for thymomas, nonthymomatous myasthenia gravis (MG) and for repeated thymectomies (rethymectomies). Some additional remarks to the standard terms and definitions will be added, as well. This seems to be indicated because in the period after publication of the Standard Terms, Definitions, and Policies the minimally-invasive thymic surgery has been developing very fast, with more than eight hundred articles (listed in PubMed) published afterwards, which brought considerable amount of new experience. Besides, not all aspects of thymectomy were analyzed in the previous article.
In the 1980s transsternal approach was a standard for thymectomy for MG. However, the 1990s brought two important changes. The first event change was the wide development of the Evidence Based Medicine (EBM) policy (introduced in 1980. at the McMaster University, Canada) (2). The use of EBM challenged the previously accepted view of the beneficial role of thymectomy in the treatment of MG (3). It has been pointed out that there were no prospective randomized trials confirming better results of thymectomy vs. medical treatment of MG. The transsternal approach started to be regarded excessively invasive for MG, the disease, that could be treated with the improved drugs much more successfully that in the past. The end of the 1980s and early 1990s was also a time of origin of minimally invasive thoracic surgery, which became an answer of the thoracic surgical community for the criticism toward a transsternal thymectomy. These were an extended transcervical technique, with elevation of the sternal manubrium and the videothoracoscopic or video-assisted (VATS) technique (4,5). The proponents of these approaches raised their advantages including a lesser burden of an operation, shorter hospitalization and recuperation and, last but not least—a better cosmesis. For these reasons and especially due to avoidance of sternotomy, these approaches, especially the VATS technique gained increasing popularity and challenged the role of transsternal thymectomy. Afterwards, many articles supporting the efficacy of minimally invasive techniques of thymectomy were published, worldwide. Finally, late results of treatment of MG were claimed to be no different from transsternal thymectomy (6,7). Nevertheless, recent results from the U.S. database suggested that hospital admissions for thymectomy in patients with nonthymomatous MG fell dramatically in this country after 2000 (8). One of the reasons might have been better immunotherapy, the other reason was a lack of prospective randomized trials supporting the role of thymectomy and the third reason (never confirmed, but suspected) an unsatisfactory effectiveness of minimally invasive techniques of thymectomy. Regarding the last objection the explanation seems simple—good results reported by experts of minimally invasive thymectomy might be not repeated so successfully by average surgeons. Whatever was the reason of decreasing number of thymectomies for nonthymomatous MG in the USA, and maybe in the other parts of the world, the results of recently published prospective randomized trial comparing extended transsternal thymectomy plus alternate-day prednisone with alternate-day prednisone alone, showing better results in the surgical arm, possibly might change positively the view of the neurologists and myasthenic patients of the role of thymectomy (8). A publication of the results of the prospective randomized trial comparing a medical treatment of MG with thymectomy provided evidence for a beneficial role of surgery performed in the extended transsternal technique. However, one must realize that the conclusions presented by Wolfe et al. do not apply as a matter of course to the minimally invasive techniques of thymectomy.
The new question appears—does the superiority of transsternal thymectomy over medical treatment is also valid for minimally invasive thymectomies?
At the present time there is no answer for this question and a new prospective randomized trial comparing transsternal and minimally invasive thymectomies has been proposed to solve the problem.
It seems probable that to achieve equally good results such a minimally invasive technique of thymectomy should be the same extensive as the transsternal one reported in the before mentioned study. The surgical pattern of technique of transsternal extended thymectomy approved for the study of Wolfe et al. was based on the technique described in detail by Jaretzki et al., which included a wide dissection of the mediastinal tissue of the lower part of the neck and the anterior mediastinum due to presence of ectopic foci of the thymic tissue dispersed in there (9). The important role of resection of the adipose tissue of the mediastinum and the lower neck for improvement of the complete remission rate after thymectomy for MG was confirmed in several previous studies (10,11). One of the conclusions of Wolfe et al. was that randomized trial to compare resectional techniques were needed (8). However, conduction of such trial would be very difficult considering a variety of techniques of minimally invasive thymectomy. In other words, it would be difficult to compare a standardized extended transsternal technique with one of the variety minimally invasive techniques of thymectomy. Analyzing this issue in detail, why such a specific technique could be chosen, instead of many others and how it could represent all the other minimally invasive techniques of thymectomy? If the results of such a technique were inferior than the results of the extended transsternal technique, would this mean that all minimally invasive techniques are inferior to the transsternal one? There are no simple solutions but the only way to find it is to analyze in detail the definitions of minimally invasive thymectomy, and what is specifically an extended thymectomy.
Methods
Definition of minimally invasive thymectomy
The minimally invasive thoracic procedures are those performed through the intercostal, subxiphoid or transcervical incisions. The last approach, nonexistent in 2011 is a subcostal one (12,13). In most of these procedures (with exception of some transcervical procedures) the video-assisted thoracic surgery (VATS) technique is used with performing operation under guidance of a video monitor. According to this definition the minimally invasive procedures do not include the sternal manubriotomy (upper sternal split), transverse sternotomy and subcutaneous longitudinal sternotomy, although in the past these procedures have been also called the minimally invasive (14-16).
Definition of an extended thymectomy
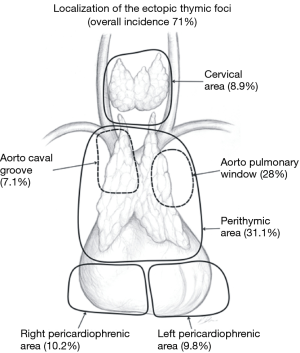
The definition of an extended thymectomy is a removal of the whole thymus with the surrounding adipose tissue. Instead of the term “extended thymectomy” some authors recommended a term “complete thymectomy”, which means total removal of the whole thymus, without mentioning of the adjacent fatty tissue (17). However, in practice, it is difficult to specify what both of these terms mean. Is a commonly performed unilateral VATS thymectomy a complete and extended one? Is a transcervical thymectomy an extended one? A detailed anatomical analysis of each individual technique is necessary to analyze these doubts. Jaretzki described an anatomical localization of possible ectopic thymic tissue in the neck and mediastinum (9). At my institution we use a simplified scheme of the areas of the adipose tissue necessary to remove during an extended or maximally extended thymectomy.
We distinguish six areas of the adipose tissue surrounding the thymus gland (Figure 1) including:
- The lower neck, around and below the thyroid gland, down to the thoracic outlet level;
- The perithymic area—extending from the upper to the lower poles of the thymus;
- The aorta-caval groove, including the right paratracheal space;
- The aorta-pulmonary window—the space between the left side of the ascending aorta, the left pulmonary artery and the left mediastinal pleura;
- The right pericardiophrenic fat—the fatty tissue below the lower poles of the thymus including the right epiphrenic fat pad;
- The left pericardiophrenic fat—the fatty tissue below the lower poles of the thymus including the left epiphrenic fat pad.
Results
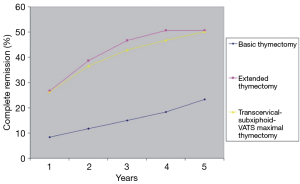
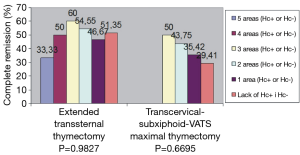
Removal of the fatty tissue from all these areas is important due to the widespread ectopic foci of the thymic tissue. The meaning of the ectopic foci and their removal is ambiguous. Some authors considered finding of the foci in the specimen as a negative predictor of complete remission (18-20). The results obtained by our group showed, that removal of 6 areas of the adipose tissue surrounding the thymus gland improved a complete remission rate in comparison with removal of the thymus gland alone in patients with nonthymomatous MG (Figure 2) (11). Our results of complete remission rate were consistent with results of Jaretzki and Masaoka (21,22). Our second and even more important finding was that the chance of remission after maximally extended thymectomy for nonthymomatous MG was still high in the presence of the ectopic foci in 3, 4 and even 5 areas of the adipose tissue of lower neck/mediastinum (Figure 3).
Discussion
Pre-conditions for the prospective randomized trial comparing transsternal extended and minimally invasive thymectomies
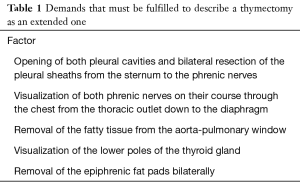
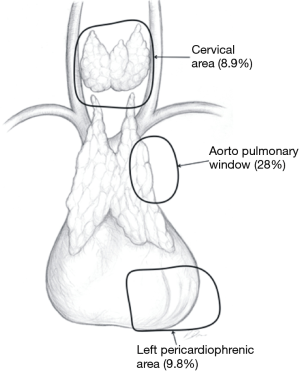
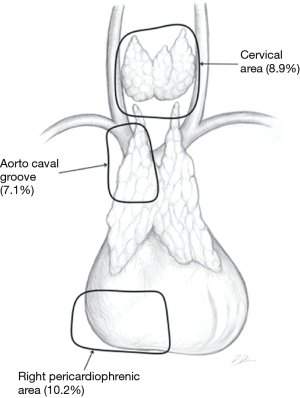
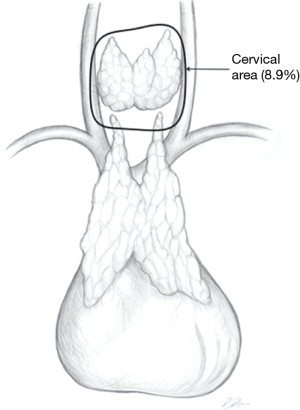
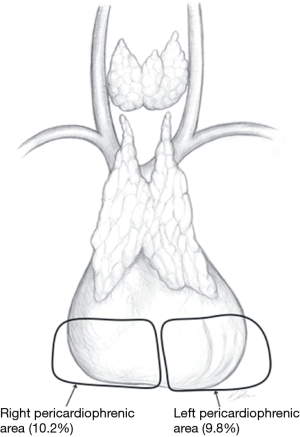
Beginning from Masaoka and Jaretzki most of thoracic surgeons became convinced about the idea of an extended thymectomy which increases the chance for postoperative improvement, or complete remission of MG due to removal of these foci, which is a prerequisite of extended thymectomy. There are several questions how to distinguish extended thymectomy from non-extended one. From the practical point of view is if the procedure including removal of the whole thymus along with some of the areas mentioned above and leaving the other not resected can be called an extended thymectomy? Or maybe a less extended one? Is it appropriate to estimate an extensiveness of thymectomy with use of weight of a specimen if the weight of the thymus and the adipose tissue of the mediastinum are so highly variable and dependent on the body habitus of a patient? Maybe the other solution is to describe in a detailed way what were the borders of resection, with specification of such information like opening of both pleural cavities, visualization of both phrenic nerves on their course through the chest from the thoracic outlet down to the diaphragm, removal of the fatty tissue from the aorta-pulmonary window, removal of the pretracheal fatty tissue up to the thyroid, visualization of the lower poles of the thyroid gland, visualization of the vagus and laryngeal recurrent nerves bilaterally and removal of the epiphrenic fat pads bilaterally (Table 1).
Full table
In the Figures 4-7 it was shown which areas of adipose tissue of the lower neck/mediastinum were omitted during most commonly performed types of minimally invasive thymectomies. It must be stressed that the technique described by Jaretzki (which was used in all patients in the surgical arm of the recent study by Wolfe et al.) included removal of the adipose tissue from all the areas mentioned above. Therefore, similar extent of dissection during a minimally invasive thymectomy should be performed to achieve results to Wolfe et al. (8). However, there are very few minimally invasive techniques of thymectomy allowing for such radical dissection. Such technique must provide possibility to fulfill all conditions listed in Table 1.
Indications for minimally invasive thymectomy in nonthymomatous MG
Nonthymomatous MG is a widely accepted indication for minimally invasive thymectomy, which is based on the presumption that this sort of technique is equally effective as an extended/maximal transsternal approach. An undisputed advantage of minimally invasive technique is a lack of sternotomy, which is an important cosmetic concern in patients’ population consisting in most part of young women.
Indications for minimally invasive thymectomy in thymoma with/without MG
Surgery for thymoma must fulfill several requirements including radical en-bloc removal of the whole tumor, non-touch technique and avoidance of violation of the tumor’s capsule. It has been recommended to remove the whole thymus considering possible multicentric growth of the tumor or to avoid possible MG appearing after thymectomy for thymoma. However, it must be clearly stated, that such events occur rarely, usually in less than 10% of patients undergoing thymectomy for thymoma without MG (23,24). On the other hand, some authors proposed subtotal thymectomy in the Masaoka stage I and II thymomas in cases without associated MG (25). However, most of the authors advice to perform a complete thymectomy in thymomas with MG. Thymomas, contrary to the thymic cancer rarely metastasize to the mediastinal lymph nodes, therefore the role of lymphadenectomy of the mediastinum for thymomas is not clear. The lymph node stations were reclassified according to the New ITMG/IASLC lymph node map (26). The anterior region (N1) included the lower anterior cervical, perithymic, prevascular, paraaortic, ascending aortic, superior phrenic, supradiaphragmatic, inferior phrenic and pericardial node groups. The deep region (N2) included the deep cervical, supraclavicular, upper and lower paratracheal, subaortic, subcarinal, hilar, and internal mammary node groups. All nodes outside the anterior and deep regions were regarded as M components (26). Hwang et al. used the new ITMG/IASLC lymph node map in the retrospective analysis of 131 patients with thymoma and thymic carcinoma and found node metastasis in 13 patients (N1 in six and N2 in seven). Six N2 patients (86%) had right paratracheal node metastases. The rates of node metastasis were 1% at T1 and 37.5% at T2/3 (P<0.001). There was no node metastasis of the A, AB, or B1 types. The authors recommended LND including RPN is recommended in stage II or higher thymic malignancies (27).
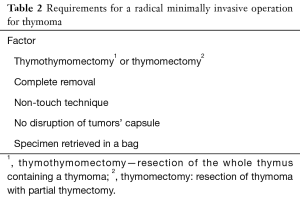
Are there any limits of the size or stage of thymoma precluding the use of minimally invasive techniques? To answer for this question one must consider the conditions, a proper operation for thymoma must fulfill the criteria listed in Table 2. The first question is what type of operation should be done for thymoma—a partial thymectomy with removal of a tumor (thymomectomy), or removal of the whole thymus containing a thymoma (thymothymomectomy or just complete thymectomy, as most of thoracic surgeons call this procedure)? There are two aspects of this problem—an oncological one and the second one regarding MG, with no randomized trials addressing any of them. Several authors found no deterioration of the survival and the recurrence rate in patients who underwent thymomectomy in comparison to thymothymomectomy (25,28,29). In contrast to these studies are the results of two large retrospective studies reported by Gu et al., and by Nakagawa et al., Gu et al., reported a large retrospective study based on the Chinese national database and found no significant difference in recurrence rates in Masaoka-Koga stage I tumors (3.2% vs. 1.4%, P=0.259) (30,31). However, in patients with Masaoka-Koga stage II tumors, recurrence was significantly less after thymectomy group than after thymomectomy (2.9% vs. 14.5%, P=0.001) (30). In another large retrospective study based on the Japanese database, Nakagawa et al. compared the results of thymectomy and thymomectomy in stage I and II thymomas. The authors found that the 5-year overall survival was similar between both groups (96.9% after thymectomy vs. 97.3% after thymomectomy). Nevertheless, there was a trend towards more local recurrences in the thymomectomy group (2.2% vs. 0.4%, P=0.0613) (31). Although the problem of choice of thymothymomectomy or thymomectomy for early-stage thymomas is not closed definitely, it seems prudent to recommend a total thymectomy as a procedure of choice in such patients. Regarding the maximal size of thymoma that can be removed by means of minimally invasive techniques any numbers cannot be specified. A radical R0 resection can be performed in cases of large stage IIIa tumors exceeding a diameter of 10 centimeters with concomitant resection of a part of the pericardium, or wedge resection of lung infiltrated marginally by the tumor.
Full table
The other issue is which approach—thymomectomy or thymothymomectomy (thymectomy) is better in regard to myasthenia. There is even less data that in case of oncological value of minimally invasive vs. transsternal thymectomy. The most important data were presented by Gu et al. in the study mentioned above (30). Improvement rate of MG was higher after thymectomy than after thymomectomy (91.6% vs. 50.0%, P<0.001), which supports the use of thymothymomectomy (thymectomy) for patients with thymoma associated with MG. There have been virtually no studies on extended vs. non-extended thymectomy for thymomas associated with MG. Our own data are based on our institutional experience with patients who underwent rethymectomy and include 8 patients with MG associated with thymoma, with 3 of them who underwent previous basic thymectomy for thymoma and the other 5 patients who underwent previous thymomectomy for thymoma. All of these patients underwent extended rethymectomy by means of minimally invasive approach (the subxiphoid—bilateral one port VATS approach with double elevation of the sternum in 6 patients and transcervical-subxiphoid-bilateral one port VATS with double elevation of the sternum in 2 patients) for deterioration of MG with the aim to remove extensively the adipose tissue of the lower neck/mediastinum. In one of these patients a part of the thymus was found, in all others the ectopic foci of the thymic tissue were discovered. In all of these patients there was a clinical improvement; however there was no complete remission in any of them. These results reassured our team to perform a maximally extended thymectomy in all patients with thymoma associated with MG, if technically feasible.
Indication for thymectomy in refractory nonthymomatous MG after previous thymectomy
The other issue which was not covered in the Standard Terms, Definitions, and Policies was indication for thymectomy for repeated thymectomy (rethymectomy) for refractory nonthymomatous MG after previous thymectomy. In this last instance it is often very difficult or even impossible to differentiate a thymic tissue from the fatty tissue with use of CT, MRI or PET/CT. Decision about rethymectomy is usually made upon clinical symptoms and discovery of the fatty tissue of the anterior mediastinum on the imaging studies. A clinical sequence of events typical for incomplete thymectomy is a temporary improvement after the first procedure with a subsequent deterioration. Rethymectomy for refractory nonthymomatous MG is a procedure currently performed rarely. Such a procedure must be differentiated from the repeated thymectomy performed for the recurrent thymoma, which is always indicated if feasible. In most of rethymectomies for nonthymomatous MG a transsternal approach is used, although two series of cases utilizing minimally approaches were reported. In one of them a left sided unilateral VATS technique was used, while a transcervical or transcervical-subxiphoid-VATS approach was used in the other one (32,33).
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Toker A, Sonett J, Zielinski M, et al. Standard Terms, Definitions, and Policies for Minimally Invasive Resection of Thymoma. J Thorac Oncol 2011;6:S1739-S1742. [Crossref] [PubMed]
- Guyatt GH, Sackett DL, Cook DJ. Users' guides to the medical literature. II. How to use an article about therapy or prevention. B. What were the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA 1994;271:59-63. [Crossref] [PubMed]
- Buckingham JM, Howard FM Jr, Bernatz PE,, et al. The value of thymectomy in myasthenia gravis. A computer-assisted matched study. Ann Surg 1976;184:453-8. [Crossref] [PubMed]
- Cooper JD, Al-Jalaihawa AN, Pearson FG,, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Landreneau RJ, Dowling RD, Castillo WM,, et al. Thoracoscopic resection of an anterior mediastinal tumor. Ann Thorac Surg 1992;54:142-4. [Crossref] [PubMed]
- Shrager JB, Deeb ME, Mick R,, et al. Transcervical thymectomy for myasthenia gravis achieves results comparable to thymectomy by sternotomy. Ann Thorac Surg 2002;74:320-6; discussion 326-7. [Crossref] [PubMed]
- Mantegazza R, Confalonieri P, Antozzi C, et al. Video-assisted thoracoscopic extended thymectomy (VATET) in myasthenia gravis Two year follow-up in 101 patients and comparison with the transsternal approach. Ann N Y Acad Sci 1998;841:749-52. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Jaretzki A, Wolff M. “Maximal” thymectomy for myasthenia gravis. Surgical anatomy and operative technique. J Thorac Cardiovasc Surg 1988;96:711-6. [PubMed]
- Masaoka A, Monden Y. Comparison of the results of transsternal simple, transcervical simple, and extended thymectomy. Ann N Y Acad Sci 1981;377:755-65. [Crossref] [PubMed]
- Zielinski M, Hauer L, Hauer J, et al. Comparison of complete remission rates after 5 year follow-up of three different techniques of thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2010;37:1137-43. [Crossref] [PubMed]
- Zhao J, Wang J, Zhao Z, et al. Subxiphoid and subcostal arch thoracoscopic extended thymectomy: a safe and feasible minimally invasive procedure for selective stage III thymomas. J Thorac Dis 2016;8:S258-64. [PubMed]
- Tang Y, Ou ZA, Liao M, et al. Subcostal thoracoscopic extended thymectomy for patients with myasthenia gravis. J Thorac Dis 2016;8:499-504. [Crossref] [PubMed]
- Otto TJ, Strugalska H. Surgical treatment for myasthenia gravis. Thorax 1987;42:199-204. [Crossref] [PubMed]
- Maggi G, Casadio C, Cavallo A, et al. Thymectomy in myasthenia gravis. Results of 662 cases operated in 15 years. Eur J Cardiothorac Surg 1989;3:504-9; discussion 510-1. [Crossref] [PubMed]
- Granone P, Margaritora S, Cesario A, et al. Focus on cosmesis in thymectomy for myasthenia gravis. Ann Thorac Surg 2001;72:1441-2. [Crossref] [PubMed]
- Calhoun RF, Ritter JH,, Guthrie TJ, et al. Results of transcervical thymectomy for myasthenia gravis in 100 consecutive patients. Ann Surg 1999;230:555-9; discussion 559-61. [Crossref] [PubMed]
- Ashour M. Prevalence of ectopic thymic tissue in myasthenia gravis and its clinical significance. J Thorac Cardiovasc Surg 1995;109:632-5. [Crossref] [PubMed]
- Ambrogi V, Mineo TC. Active ectopic thymus predicts poor outcome after thymectomy in class III myasthenia gravis. J Thorac Cardiovasc Surg 2012;143:601-6. [Crossref] [PubMed]
- Ponseti JM, Gamez J, Vilallonga R, et al. Influence of ectopic thymic tissue on clinical outcome following extended thymectomy in generalized seropositive nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2008;34:1062-7. [Crossref] [PubMed]
- Jaretzki A. Thymectomy for myasthenia gravis: analysis of the controversies regarding technique and results. Neurology 1997;48:S52-63. [Crossref]
- Masaoka A, Yamakawa Y, Niwa H, et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg 1996;62:853-9. [Crossref] [PubMed]
- Kondo K, Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg 2005;28:22-5. [Crossref] [PubMed]
- Yamada Y, Yoshida S, Iwata T, et al. Risk factors for developing postthymectomy myasthenia gravis in thymoma patients. Ann Thorac Surg 2015;99:1013-9. [Crossref] [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [Crossref] [PubMed]
- Bhora FY, Chen DJ, Detterbeck FC, et al. The ITMIG/IASLC Thymic Epithelial Tumors Staging Project: a proposed lymph node map for thymic epithelial tumors in the forthcoming 8th edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S88-S96.
- Hwang Y, Park IK, Park S, et al. Lymph Node Dissection in Thymic Malignancies: Implication of the ITMIG Lymph Node Map, TNM Stage Classification, and Recommendations. J Thorac Oncol 2016;11:108-14. [Crossref] [PubMed]
- Narm KS, Lee CY, Do YW, et al. Korea Association for Research on the Thymus. Limited thymectomy as a potential alternative treatment option for early-stage thymoma: A multi-institutional propensity-matched study. Lung Cancer 2016;101:22-7. [Crossref] [PubMed]
- Tseng YC, Hsieh CC, Huang HY, et al. Is thymectomy necessary in nonmyasthenic patients with early thymoma? J Thorac Oncol 2013;8:952-8. [Crossref] [PubMed]
- Gu Z, Fu J, Shen Y, et al. Members of the Chinese Alliance for Research in Thymomas. Thymectomy versus tumor resection for early-stage thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis. J Thorac Dis 2016;8:680-6. [Crossref] [PubMed]
- Nakagawa K, Yokoi K, Nakajima J, et al. Is Thymomectomy Alone Appropriate for Stage I (T1N0M0) Thymoma? Results of a Propensity-Score Analysis. Ann Thorac Surg 2016;101:520-6. [Crossref] [PubMed]
- Pompeo E, Nofroni I, Iavicoli N, et al. Thoracoscopic Completion thymectomy in refractory nothymomatous myasthenia. Ann Thorac Surg 2000;70:918-23. [Crossref] [PubMed]
- Zieliński M. Rethymectomy in the treatment of refractory myasthenia gravis: operative technique through the transcervical approach. In: Zielinski M, Rami-Porta R. editors. The transcrvical approach in thoracic surgery. Springer-Verlag Berliin Heidelberg, 2014;137-40.
Cite this article as: Zieliński M. Definitions and standard indications of minimally-invasive techniques in thymic surgery. J Vis Surg 2017;3:99.