Minimally invasive surgical procedures for thymic disease in Asia
The dawn of video-assisted thoracic surgery (VATS) for thymic diseases in Asia
Prior to development of VATS procedures, an extended thymectomy for myasthenia gravis and resection of thymic tumors were commonly performed via a median sternotomy, while a cervical thymectomy was adopted by some surgeons because of its lower level of invasiveness. Cooper et al. presented a novel retractor for lifting the sternum to facilitate a wide view of the entire thymus through an anterior cervical incision (1). Unfortunately, that procedure did not become common or standard because of poor consensus regarding complete resection of the caudal portion of the thymus. However, the situation was dramatically changed by introduction of endoscopic surgical techniques in the early 1990s.
VATS was initially adopted for simple procedures performed unilaterally in the thoracic cavity, such as wedge resection of the lung, treatment of pneumothorax, and resection of a posterior mediastinal tumor. In contrast to lung resection, a VATS approach was initially not considered suitable for a thymectomy, because the anterior mediastinal space is narrow for manipulation and also because an extended thymectomy requires procedures in the bilateral thoracic cavity. In addition, techniques needed to safely ligate and divide the thymic veins were also considered difficult and sometimes rather dangerous to perform in the narrow space of the anterior mediastinum.
In the middle 1990s, several surgeons in various countries nearly simultaneously tackled these issues. Yim et al. in Hong Kong began performing a VATS extended thymectomy for myasthenia gravis in 1993 and reported their experience with eight cases in 1995 (2). In Japan, Andou et al. also reported two cases of VATS extended thymectomy with a cervical incision in 1996 (3), while Cheng et al. in Taiwan began performing resection of thymic tumors including thymoma with or without myasthenia gravis in 1999, and reported their experience with seven cases (4). Thus, Asian surgeons were performing pioneering work prior to 2000, though the largest series of cases was reported in 1996 in the United States.
Technical progress of VATS thymectomy in Asia
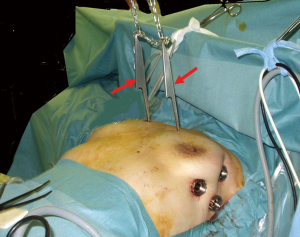
A variety of techniques for use of VATS have been proposed, with the most common thought to be a bilateral thoracoscopic approach, though some surgeons prefer a unilateral thoracoscopic or infra-sternal approach. With all of the early reported methods, the narrow view of the anterior mediastinum was a common difficulty. This problem was solved by lifting the sternum, a method initially developed by the efforts of several Japanese surgeons. Kido et al. first presented a technique for lifting the sternum by use of a Laparolift (Origin, Co., Ltd., CA, USA) in combination with an infra-sternal approach in 1999 (5). Thereafter, Uchiyama et al. used the same method and reported their experience with 23 cases in 2001 (6). Takeo et al. then introduced a method for lifting the sternum with 10-mm cotton string behind the sternum by inserting from the anterior neck to the subxiphoid region (7), while Zieliński et al. in Poland independently developed a similar method for lifting the sternum (8). Next, Ohta et al. reported a novel costal hook, and introduced a method for lifting the bilateral third ribs and anterior chest wall as shown in Figure 1 (9), which was improved by Shiono et al. (10).
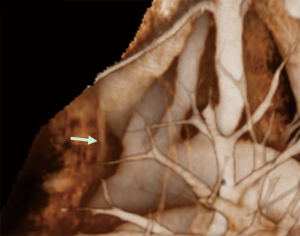
Another important topic related to performance of a VATS thymectomy is safety for dissection and division of the thymic veins. Shiono et al. utilized preoperative multidetector-row computed tomography (MDCT) and reconstructed 3D images of the thymic veins to visualize their location, number, and branching pattern (11), which clearly identified the thymic veins draining into the LBV as shown in Figure 2. It was found that such 3D imaging of the thymic veins was helpful for navigation during the operation and increased the safety of VATS thymectomy procedures.
There has been controversy regarding the necessity of a cervical incision for VATS thymectomy procedures. Shigemura et al. examined the size of the residual thymus in the anterior cervical region in cases without cervical incision and concluded that VATS thymectomy without a transcervical approach may be an immunologically incomplete treatment for myasthenia gravis (12). Although that study clearly showed that a transcervical approach is essential for VATS thymectomy to ensure its radicalness, progress in technology might bring about alterations in the approach. For example, CO2 insufflation in the thoracic cavity has recently been utilized for VATS in a manner similar to laparoscopic surgery. Furthermore, we have found that dividing the right intrathoracic vein provides a wide view of the anterior upper mediastinum and anterior cervical regions. Such technical progression has reduced the necessity of cervical incision as well as lifting of the sternum. A recent bilateral thoracoscopic extended thymectomy procedure performed at Osaka University can be seen in a video (Figure 3).

Recently, Suda et al. in Japan introduced a single-port thymectomy technique performed through a subxiphoid incision and reported the superiority of this approach in comparison to a conventional VATS method in regard to duration of hospital stay, and amount and duration of postoperative oral analgesics (14).
Survey of VATS performed for thymic disease in Asia
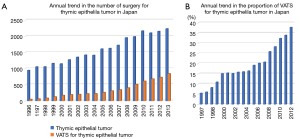
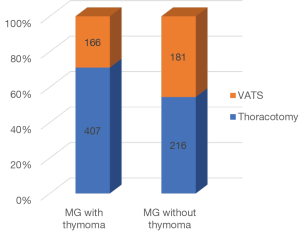
The Japanese Association for Thoracic Surgery (JATS) has been surveying the number of cardiac and general thoracic surgical operations performed each year throughout Japan since 1986, and added items in regard to VATS performed for the lung and mediastinum starting in 1996 (15-17). Trends for all types of surgery for thymic epithelial tumors and the number of VATS procedures are presented in Figure 4A, which indicates a continuous increase in number of VATS cases. The proportion of VATS procedures among surgery cases for thymic epithelial tumors is shown in Figure 4B. Interestingly, the proportion of VATS was constant from 2000 to 2005, then began to increase from 2006. This might be related to introduction of several methods for lifting the sternum in Japan as well as development of energy devices. A difference has been found in VATS extended thymectomy procedures for myasthenia gravis according to the association with a thymoma, as JATS survey results from 2012 and 2013 revealed that a VATS extended thymectomy was performed in 29% of patients with and in 46% of those without a thymoma (Figure 5).
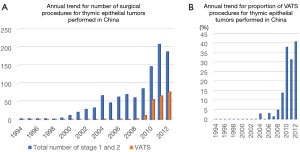
A search of the database established by the Chinese Alliance for Research in Thymomas (ChART) also revealed that the annual number of VATS procedures for thymic epithelial tumors has increased (18). That retrospective database shows that VATS was introduced in China in 2004, 10 years later as compared to Japan, though the number of patients treated has thereafter steadily increased as shown in Figure 6A. Notably, the number of VATS cases rapidly rose after 2010 and reached 41% in 2012 (Figure 6B), similar to Japan.
Outcome of VATS performed for thymic diseases in Asia
In 2005, Manlulu et al. reported long-term outcomes of VATS thymectomy procedures performed in Hong Kong for non-thymomatous myasthenia gravis based on their 12 years of experience with 38 patients. Improvement was observed in 91.6% of those cases and complete stable remission was achieved in 22.2% after a median follow-up period of 69 months (19).
Shiono et al. reported the outcomes of a bilateral extended thymectomy with anterior chest wall lifting performed in Japan for 30 patients with myasthenia gravis without thymoma association, and then compared those results with a historical control group that underwent a thymectomy through a median sternotomy at the same institute performed by the same group between 1996 and 2000. The median weight of the removed tissues was 37.0 g in the VATS group, which was not significantly different from that obtained by a trans-sternal extended thymectomy (34.0 g). Furthermore, palliation and remission rates at 3 years after the thymectomy were 88% and 43%, respectively, and there was no difference as compared with the historical control group (10).
In a study conducted in Iran, Toolabi et al. reported mid-term outcomes of right-sided VATS for 31 myasthenia gravis patients. They noted that 27 patients (87%) showed clinical improvement and 11 (35%) had complete remission during the mean follow-up period of 20 months (20).
Lee et al. also retrospectively reviewed their cases of VATS extended thymectomy (55 patients) and extended thymectomy through median sternotomy (59 patients) performed in South Korea from 2006 to 2009, and found no differences in regard to the weight of removed tissue (21).
In a review of cases in the ChART database, Wang et al. reported survival for 1,117 thymoma patients who underwent surgery for clinically early-stage (Masaoka-Koga stage I and II) disease between 1994 and 2012. There were 241 patients treated by VATS and their 5-year overall survival was 92%, which was not significantly different as compared with the 5-year overall survival of patients who underwent an open thymectomy (18).
As those reports show, the number of annual VATS procedures is increasing in many countries and areas, though long-term survival after more than 10 years should also be investigated, especially for discussion of the outcome of such treatment for a thymoma. Until a better understanding of the long-term effects can be attained, care should be taken prior to extension of the indications of VATS, for example, in cases with a large sized tumor. Recently, Kimura et al. reported regarding the risk of postoperative pleural recurrence in cases with a thymoma greater than 5 cm or with a large cystic portion (22).
Robot-assisted thoracoscopic surgery for thymic diseases in Asia
Yoshino et al. was the first to report resection of a thymic neoplasm by Da Vinci robot-assisted thoracoscopic surgery, which was performed in Japan (23). Nakamura et al. thereafter reviewed 112 cases of Da Vinci robot-assisted thoracoscopic surgery in Japan performed through September 2012 at nine institutions (24). Among those, 38 patients had anterior-middle mediastinal disease including 18 with a thymoma. The surgical procedure was tumor excision in 15 and thymectomy in 23. The durations of postoperative and total hospital stays were 7.1 and 12.8 days, respectively, and no patient died and no recurrence had been seen in any patient at the time of writing. Unfortunately, the number of cases is quite small because of restrictions related to national health insurance coverage. Nevertheless, robotic surgery has been safely introduced in Japan.
Early outcomes of robot-assisted surgery for the anterior mediastinum performed in South Korea were reported by Seong et al. (25). Propensity score matching was done to compare with a thoracotomy, which revealed a lower number of drains, lower 24-hour tube drainage amounts, less hemoglobin loss, fewer chest tube days, and shorter length of hospital stay in the robotic group.
In Japan, Suda et al. utilized a da Vinci surgical system for their single-port thymectomy method performed through a subxiphoid incision and reported experience with eight cases of single-port thymectomy (26). The early outcome of robot-assisted thoracoscopic surgery was shown to be equal to that of single-port thymectomy cases and the authors recommended favorable indication for a robot-assisted thymectomy procedure when the tumor showed invasion to surrounding organs.
Concluding remarks
Asian surgeons have played leading roles in minimally invasive thoracoscopic surgery for the thymus and the number of patients undergoing VATS for thymic diseases is dramatically increasing. In addition, advances have been made with robot-assisted thoracoscopic surgery, though some development remains. Continued progress in minimally invasive surgery for thymic diseases in Asia is anticipated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Yim AP, Kay RL, Ho JK. Video-assisted thoracoscopic thymectomy for myasthenia gravis. Chest 1995;108:1440-3. [Crossref] [PubMed]
- Andou A, Azuma T, Tsukazaki T, et al. Thoracoscopic extended thymectomy with collar incision of the neck in two patients with myasthenia gravis. J Jpn Assoc Chest Surg 1996;10:107-11. [Crossref]
- Cheng YJ, Wu HH, Chou SH, et al. Video-assisted thoracoscopic management of mediastinal tumors. JSLS 2001;5:241-4. [PubMed]
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [Crossref] [PubMed]
- Uchiyama A, Shimizu S, Murai H, et al. Infrasternal mediastinoscopic thymectomy in myasthenia gravis: surgical results in 23 patients. Ann Thorac Surg 2001;72:1902-5. [Crossref] [PubMed]
- Takeo S, Sakada T, Yano T. Video-assisted extended thymectomy in patients with thymoma by lifting the sternum. Ann Thorac Surg 2001;71:1721-3. [Crossref] [PubMed]
- Zieliński M, Kuzdzał J, Szlubowski A, et al. Transcervical-subxiphoid-videothoracoscopic "maximal" thymectomy—operative technique and early results. Ann Thorac Surg 2004;78:404-9. [Crossref] [PubMed]
- Ohta M, Hirabayasi H, Okumura M, et al. Thoracoscopic thymectomy using anterior chest wall lifting method. Ann Thorac Surg 2003;76:1310-1. [Crossref] [PubMed]
- Shiono H, Kadota Y, Hayashi A, et al. Comparison of outcomes after extended thymectomy for myasthenia gravis: bilateral thoracoscopic approach versus sternotomy. Surg Laparosc Endosc Percutan Tech 2009;19:424-7. [Crossref] [PubMed]
- Shiono H, Inoue A, Tomiyama N, et al. Safer video-assisted thoracoscopic thymectomy after location of thymic veins with multidetector computed tomography. Surg Endosc 2006;20:1419-22. [Crossref] [PubMed]
- Shigemura N, Shiono H, Inoue M, et al. Inclusion of the transcervical approach in video-assisted thoracoscopic extended thymectomy (VATET) for myasthenia gravis: a prospective trial. Surg Endosc 2006;20:1614-8. [Crossref] [PubMed]
- Okumura M, Shintani Y, Ohta M, et al. Extended thymectomy by bilateral VATS. Asvide 2017;4:308. Available online: http://www.asvide.com/articles/1619
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-8. [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Masuda M, et al. Thoracic and cardiovascular surgery in Japan during 2012: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014;62:734-64. [Crossref] [PubMed]
- Committee for Scientific Affairs., Masuda M, et al. The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan during 2013: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2015;63:670-701. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Masuda M, et al. Thoracic and cardiovascular surgery in Japan during 2014: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2016;64:665-97. [Crossref] [PubMed]
- Wang H, Gu Z, Ding J, et al. Perioperative outcomes and long-term survival in clinically early-stage thymic malignancies: video-assisted thoracoscopic thymectomy versus open approaches. J Thorac Dis 2016;8:673-9. [Crossref] [PubMed]
- Manlulu A, Lee TW, Wan I, et al. Video-assisted thoracic surgery thymectomy for nonthymomatous myasthenia gravis. Chest 2005;128:3454-60. [Crossref] [PubMed]
- Toolabi K, Aminian A, Javid MJ, et al. Mid-term results of thoracoscopic thymectomy for myasthenia gravis. Neurol India 2009;57:402-5. [Crossref] [PubMed]
- Lee CY, Kim DJ, Lee JG, et al. Bilateral video-assisted thoracoscopic thymectomy has a surgical extent similar to that of transsternal extended thymectomy with more favorable early surgical outcomes for myasthenia gravis patients. Surg Endosc 2011;25:849-54. [Crossref] [PubMed]
- Kimura T, Inoue M, Kadota Y, et al. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [Crossref] [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 2001;122:783-5. [Crossref] [PubMed]
- Nakamura H, Suda T, Ikeda N, et al. Initial results of robot-assisted thoracoscopic surgery in Japan. Gen Thorac Cardiovasc Surg 2014;62:720-5. [Crossref] [PubMed]
- Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion e73.
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
Cite this article as: Okumura M, Shintani Y, Ohta M, Kadota Y, Inoue M, Shiono H. Minimally invasive surgical procedures for thymic disease in Asia. J Vis Surg 2017;3:96.