Prevention and management of intraoperative crisis in VATS and open chest surgery: how to avoid emergency conversion
Introduction
Video assisted thoracic surgery (VATS) has become a frequently utilized approach for an increasingly wide and complex range of diagnostic and therapeutic procedures of the chest. It has been associated with decreased postoperative pain, shorter length of stay and lower incidence of complications such as pneumonia. Limitations to this modality may include limited exposure, lack of tactile feedback, and a two-dimensional view of the surgical field. Furthermore, the lack of an open incision may incur technical challenges in preventing and controlling operative misadventures leading to major hemorrhage or other intraoperative emergencies. Given the nature of the vascular anatomy and physiology of the chest, injury due to unrecognized variants combined with high rates of blood flow within a low pressure system can lead to catastrophic hemorrhage within moments. While these events may occur in the best of circumstances, prevention strategies are the primary means of avoiding these injuries. When they do occur, premeditated and expedited intervention is the key to rescue (1). The current report focuses on the prevention and management of these emergency bleeding events in VATS pulmonary resections.
Preoperative radiologic assessment of vascular and anatomic variation
Rigorous preoperative evaluation of fitness of surgery is a key component of any operative assessment. Preoperative identification and awareness of case-specific and patient-specific risk factors may help prevent hemorrhagic complications in most lung resections. These factors include body habitus, preoperative chemotherapy and radiation, bleeding diatheses including medical anticoagulants, previous thoracic surgery, lymph node calcification and/or granulomatous disease, tumor size and location.
Computed chest tomography has become a standard of care in virtually all cases considered for major pulmonary resection. These high resolution scans, often available with three dimensional reconstruction, can provide valuable opportunities to identify standard and anomalous vascular variations prior to surgery in greater than 95% of cases (2-6). Imaging may also identify calcification of lymph nodes, which may predict a conversion rate of greater than 30% when hilar or central in location (3).
Pulmonary arterial vascular patterns vary widely and may often lead to bleeding complications when not recognized. These can readily be identified on preoperative imaging. Variation should be considered the “norm”, particularly with patterns to the upper lobes, where the origin of the posterior ascending artery on the right and the number of vessels on the left may vary significantly. Venous anomalies are less common, but equally important to recognize. While the most common venous variation identified in 3–6% of cases is location of a segmental vein traversing posterior to the bronchus intermedius (which must be recognized during subcarinal lymph node dissection), up to 30% of patients may have significant venous variation, including a common ostium of the upper and lower lobe veins, particularly on the left (7-9). Given this significant degree of variability, recommendations from some authors have advised routine formal preoperative assessment of venous pulmonary vasculature with computed tomography prior to all resections (10). Clearly, whether to approach a lung resection via an open versus a thoracoscopic technique is multifactorial. Ultimately, the surgeon’s experience and level of comfort with minimally invasive techniques is what determines the choice of surgical approach in the majority of cases.
General VATS imaging challenges
Misadventures during VATS are often attributed to the two-dimensional view afforded by most common imaging camera systems. Adaption to this lack of depth perception occurs with experience through a number of conscious and subconscious mechanisms and techniques including object interposition, relative scales of motion between objects, alteration between near and far views, alteration between views through different ports, shadowing, assessment of texture variation and gradients, and knowledge of known object sizes. Combined, these mechanisms can closely approximate a three-dimensional view to the experienced surgeon (11-14). Measures that may predict successful outcomes in cases of bleeding or other complications include the designation of a dedicated thoracic surgical team and the availability of appropriate instrumentation and equipment at the time of emergency (15-20). Routine verbalization of a preoperative emergency check list, or “time-out”, specifically detailing procedural responsibilities of all members of the operative team, as well as confirming immediate availability of requisite emergency equipment, may serve to better ensure smooth execution of necessary intervention in the rare instances that catastrophic complications occur.
Intraoperative bleeding: basic principles
Once bleeding occurs, the primary goal is immediate control of hemorrhage and reestablishing view of the operative field. Ideally, this is performed with direct compression (most often) or vascular clamping. Packing of the chest through minimally invasive access incisions may also help temporize severe bleeding. If adequate hemostasis is accomplished, an assessment of need for conversion to repair can be made. If necessary, this additional time should be used to obtain appropriate blood products, maximize resuscitation of the patient, obtain necessary monitoring and venous access lines, identify and obtain additional needed assistance and equipment, and plan next steps to proceed. Equally important, this respite may serve to reestablish a calm and controlled atmosphere in which to proceed. As mentioned above, routine preparation and iteration of an emergency “time-out” prior to all cases may better prepare the operative team to facilitate a smooth, calm, and efficient process during urgent/emergent events and conversion.
Basic principles during these bleeding events consist of obtaining proximal and distal vascular control, assessing the nature and extent of injury, and executing repair or other necessary interventions. Generally, injuries less than 30% of the vessel circumference can be repaired primarily. Injuries between 30% and 50% of the circumference may be patch repaired with elements such as vein, pericardium, or prosthetic materials. Injuries involving more than 50% of the circumference will usually require end-to-end anastomosis (if length is adequate), conduit interposition, or ligation. Minor vasculature or parenchymal injuries often may be addressed adequately with a period of applied pressure. Common methods of pressure application include use of a sponge on a clamp or “stick” (most common), suction-compression, and closure of the surgical stapler on the bleeding source. Electrocautery, hemostatic clips, and other hemostatic agents may be utilized as well. Proximal vascular control is generally obtained around the main pulmonary arteries and can be accomplished within the pericardium, if needed, for proximal or complex injuries. Distal control occasionally can be obtained directly on the artery or vein of interest, but generally is performed at the level of the pulmonary veins, and also can be performed intrapericardial if needed. Cardiopulmonary bypass or extracorporeal membrane oxygenation may also be considered if the injury is felt not to be easily or safely approached without circulatory isolation of the lungs.
VATS lung resections: intraoperative bleeding and other complications
Overall, open and minimally invasive lung resections have been associated with low operative mortality and low incidence of intraoperative bleeding. A national database with over 33,000 patients found an incidence of intraoperative bleeding of 1.9% for open, 1.3% for VATS and 1.7% for robotic-assisted lobectomy resections (21). Single institutional series have reported conversion rates to open thoracotomy during robotic-assisted lobectomy in approximately 1–2% of cases (17,22). A series with 1,100 VATS anatomic lung resections reported seven conversions due to intraoperative bleeding and no intraoperative deaths (23). In a large literature review, robotic-assisted lobectomy was comparable to VATS, with conversion of robotic cases to open procedures reported in 1–19.2% (mean 8.7%) of cases (24,25). A single institution series of over 600 VATS lobectomies from Memorial Sloan-Kettering Cancer Center reported on 12 cases of “catastrophic” complications requiring conversion, emergent return to the operating room, and/or additional rescue procedures, including pneumonectomy (26). Injuries described included pulmonary arterial injury, inadvertent ligation and division of the right main pulmonary artery, division of a common pulmonary vein during left upper lobectomy, dehiscence of a pulmonary vein staple line, and injury to the azygo-caval junction. Subdiaphragmatic injury to both liver and spleen have been described secondary to trocar placement during these procedures as well (27). Rare cases of pericardial tamponade have been reported secondary to bleeding and retraction of vessels into the pericardium, and direct injury to coronary vessels (28-31).
Device or stapler failure leading to bleeding events are relatively rare, with most occurrences likely related to operator error. In a review of the Manufacturer and User Facility Device Experience (MAUDE) reporting system, 21 deaths during thoracic surgical procedures resulting from device malfunction were reported from over 17,500 reports filed from 1992–2001 (32). Most common reasons for device malfunction included failure of staples to form, general device failure or malfunction, suture line separation, stapler not firing properly, and sticking. Significant oozing from staple lines, laceration of vascular structures during stapler compression, technical injury during stapler insertion, failure of stapler release, and sharp division without staple deployment have also been reported (33,34).
Surgical prevention and management techniques
Appropriate and clear exposure and identification of critical anatomic structures combined with attention to careful and meticulous vascular dissection, ligation, and division are the most critical elements in avoiding emergent injuries. Vascular injury during introduction of staplers is one of the most frequently involved mechanisms of major injury. During stapler deployment, the surgeon should consider avoiding application of clips that may prohibit proper stapler firing, careful stapler alignment, adequate dissection and exposure around vessels to maximize unrestricted stapler passage. Tension and traction during device deployment are key components in preventing injury, which often can occur at branching points (16,18). Described techniques to facilitate safe stapler passage include use of a red rubber catheter or penrose drain to guide the anvil, or use of silk sutures or vessel loops to maximize the space for anvil passage around the vessels (15,35,36).
When minor bleeding occurs, including oozing from staple lines, small vessel injury, or parenchymal injuries, these may often be managed with a period of applied pressure. Methods of tamponade may include sponges on a clamp or “stick”, use of the lung tissue to compress injuries, suction compression, and closure of staplers on the bleeding source (15,20,23,34,37,38). Use of electrocautery, clips, and other hemostatic agents may also be useful.
Proximal vascular control should always at least be considered, and is most often obtained around the main pulmonary arteries and can be accomplished intrapericardial if needed for proximal or complex injuries. Distal control occasionally can be obtained directly on the artery or vein of interest, but generally is performed at the level of the pulmonary veins, and can also be performed intrapericardial if needed. Minimally invasive vascular control of the pulmonary artery with suture, clamps, or vessel loops has been described and may be considered dependent on the experience of the surgeon (39-42).
Once bleeding occurs, the primary goal is control and stabilization of hemorrhage. Once controlled, assessment of the injury and determination of the type and manner of repair or salvage can be made. Standard options for vascular injury repair are primary closure, patch closure, end-to-end anastomosis, and use of conduit. Primary repair is generally reserved for injuries less than one third of the circumference of the vessel. Commonly described tissue options for patch closure include autologous pericardium, bovine pericardium, and azygous vein. Conduit options include autologous pericardium, pulmonary vein, or PTFE. In the presence of concomitant airway injuries, use of interposition muscle flaps may be considered to prevent bronchoarterial fistulas (43-47). Complex repair of the pulmonary vein is most commonly associated with stapling of a common trunk of the left pulmonary vein. Described repairs include primary end to end anastomosis, use of vein cuff from the pulmonary vein of the resected lobe, and autologous pericardial patch (26,48,49). While minimally invasive approaches to these repairs have been described, the majority of complex repairs are performed by thoracotomy (50,51).
Conversion to open procedures
Conversion should generally be considered the more “conservative” measure, with a low threshold to proceed with repair in the most expeditious and safe manner based on the experience and skill of the surgeon. If repair is required and to be approached minimally invasively, skill with intracorporeal vessel isolation, suturing, and tying are generally desired. If control of hemorrhage cannot be established, immediate conversion is generally mandated to avoid catastrophic exsanguination when possible. In all situations, the approach to the open procedure should take into account the best exposure for repair of the injury, as well as completion of the index procedure. In the vast majority of VATS pulmonary resections, this is likely to be a posterolateral or anterolateral thoracotomy on the ipsilateral operative side. Consideration should be given to the availability and need for cardiopulmonary bypass for more severe or central injuries. Extracorporeal membrane oxygenation, if available, can also be considered for severe airway or vascular injuries combined with concomitant difficulties with ventilation or oxygenation. The majority of injuries can be managed without these measures.
Conversion to open thoracotomy or other approaches has been reported to range from 2% to 15% in most modern series, and is strongly predicted by the experience of the surgeon (Table 1) (15,16,23,25,52-60). While most often conversions are driven by vascular or bronchial injuries, the number of planned conversions in order to avoid catastrophic injuries represents a large portion of these cases as well. Once the decision to proceed with an open operation is made, maintaining the thoracoscopic view to ensure ongoing hemostasis while incision is made may be beneficial.
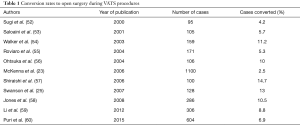
Full table
Short and long term outcomes in patients converted to open procedures during minimally invasive lung resection seem to not be adversely effected. In one report, no adverse outcomes were seen in 30 patients converted to open from a series of 286 patients in whom VATS was the initial approach for cancer (58). Eleven of these conversions were for bleeding (37%) and 2 for stapler misfire (7%).
While two other reports did identify increased operating times, blood loss, and length of stay in converted patients, there were no differences in post-operative complication, survival and recurrence rates (61,62).
Conclusions
Unplanned conversions for major intraoperative bleeding or airway injury during general thoracic surgical procedures are relatively rare and often can be avoided with careful preoperative planning, review of relevant imaging, and meticulous surgical technique. When these events occur, a pre-planned, methodical response with initial control of bleeding, assessment of injury, and appropriate repair and/or salvage procedures are necessary to maximize outcomes. During open operations, damage and hemorrhage control techniques may be more readily applied through the existing incision than during VATS operations, including immediate compression, vascular control, and if necessary, packing of the chest. During VATS operations requiring conversion to open surgery, the surgeon should be well versed in injury-specific incisions and approaches to maximize adequate exposure and, when feasible, allow completion of the index operation. Decisions to continue minimally invasively should consider the comfort and experience level of the surgeon with these techniques, and the relative benefit gained against the risk incurred to the patient. These algorithms may shift with increasing sophistication and capabilities of minimally invasive technologies and approaches.
Acknowledgments
Ms. Kathy E. Lovas, editorial support and manuscript preparation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Villa M, Sarkaria IS. Great Vessel Injury in Thoracic Surgery. Thorac Surg Clin 2015;25:261-78. [Crossref] [PubMed]
- Watanabe S, Arai K, Watanabe T, et al. Use of three-dimensional computed tomographic angiography of pulmonary vessels for lung resections. Ann Thorac Surg 2003;75:388-92; discussion 92. [Crossref] [PubMed]
- Endo S, Tsubochi H, Nakano T, et al. A dangerous venous variation in thoracoscopic right lower lobectomy. Ann Thorac Surg 2009;87:e9-10. [Crossref] [PubMed]
- Akiba T, Marushima H, Harada J, et al. Importance of preoperative imaging with 64-row three-dimensional multidetector computed tomography for safer video-assisted thoracic surgery in lung cancer. Surg Today 2009;39:844-7. [Crossref] [PubMed]
- Fukuhara K, Akashi A, Nakane S, et al. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg 2008;34:875-7. [Crossref] [PubMed]
- Akiba T, Marushima H, Harada J, et al. Anomalous pulmonary vein detected using three-dimensional computed tomography in a patient with lung cancer undergoing thoracoscopic lobectomy. Gen Thorac Cardiovasc Surg 2008;56:413-6. [Crossref] [PubMed]
- Asai K, Urabe N, Yajima K, et al. Right upper lobe venous drainage posterior to the bronchus intermedius: preoperative identification by computed tomography. Ann Thorac Surg 2005;79:1866-71. [Crossref] [PubMed]
- Kim YH, Marom EM, Herndon JE 2nd, et al. Pulmonary vein diameter, cross-sectional area, and shape: CT analysis. Radiology 2005;235:43-9; discussion 9-50. [Crossref] [PubMed]
- Akiba T, Marushima H, Odaka M, et al. Pulmonary vein analysis using three-dimensional computed tomography angiography for thoracic surgery. Gen Thorac Cardiovasc Surg 2010;58:331-5. [Crossref] [PubMed]
- Sugimoto S, Yamashita A, Baba M, et al. Pericardial drainage prior to operation contributes to surgical repair of traumatic cardiac injury. Jpn J Thorac Cardiovasc Surg 1999;47:31-5. [Crossref] [PubMed]
- Liu F, Shen C, Lin G, et al. Learning Depth from Single Monocular Images Using Deep Convolutional Neural Fields. IEEE Trans Pattern Anal Mach Intell 2016;38:2024-39. [Crossref] [PubMed]
- Kumar A, Wang YY, Wu CJ, et al. Stereoscopic visualization of laparoscope image using depth information from 3D model. Comput Methods Programs Biomed 2014;113:862-8. [Crossref] [PubMed]
- Dion YM, Gaillard F. Visual integration of data and basic motor skills under laparoscopy. Influence of 2-D and 3-D video-camera systems. Surg Endosc 1997;11:995-1000. [Crossref] [PubMed]
- Voorhorst FA, Overbeeke KJ, Smets GJ. Using movement parallax for 3D laparoscopy. Med Prog Technol 1996-1997;21:211-8. [Crossref] [PubMed]
- Demmy TL, James TA, Swanson SJ, et al. Troubleshooting video-assisted thoracic surgery lobectomy. Ann Thorac Surg 2005;79:1744-52; discussion 53.
- Berry MF, D'Amico TA. Complications of thoracoscopic pulmonary resection. Semin Thorac Cardiovasc Surg 2007;19:350-4. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5 Suppl 3:S182-9. [PubMed]
- Cerfolio RJ, Bryant AS. How to teach robotic pulmonary resection. Semin Thorac Cardiovasc Surg 2013;25:76-82. [Crossref] [PubMed]
- Xiao ZL, Mei JD, Pu Q, et al. Technical strategy for dealing with bleeding during thoracoscopic lung surgery. Ann Cardiothorac Surg 2014;3:213-5. [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 42-4. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 5-6. [Crossref] [PubMed]
- Nakamura H.. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2014;20:93-8. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg 2011;142:1412-7. [Crossref] [PubMed]
- Augustin F, Schmid T, Bodner J. The robotic approach for mediastinal lesions. Int J Med Robot 2006;2:262-70. [Crossref] [PubMed]
- Tovar EA. Pulmonary resection complicated by abrupt pericardial tamponade. Ann Thorac Surg 1995;60:1864. [Crossref] [PubMed]
- McLean RH, Parandian BB, Nam MH. Pericardial tamponade: an unusual complication of lobectomy for lung cancer. Ann Thorac Surg 1999;67:545-6. [Crossref] [PubMed]
- Ozawa Y, Ichimura H, Sato T, et al. Cardiac tamponade due to coronary artery rupture after pulmonary resection. Ann Thorac Surg 2013;96:e97-9. [Crossref] [PubMed]
- Chen J, Chen Z, Pang L, et al. A malformed staple causing cardiac tamponade after lobectomy. Ann Thorac Surg 2012;94:2107-8. [Crossref] [PubMed]
- Brown SL, Woo EK. Surgical stapler-associated fatalities and adverse events reported to the Food and Drug Administration. J Am Coll Surg 2004;199:374-81. [Crossref] [PubMed]
- Yim AP, Ho JK. Malfunctioning of vascular staple cutter during thoracoscopic lobectomy. J Thorac Cardiovasc Surg 1995;109:1252. [Crossref] [PubMed]
- Craig SR, Walker WS. Potential complications of vascular stapling in thoracoscopic pulmonary resection. Ann Thorac Surg 1995;59:736-7; discussion 7-8. [Crossref] [PubMed]
- Lin MW, Lee JM, Lee YC. Penrose drain tube as a guide for endostaplers during lobectomy via video-assisted thoracoscopic surgery. Thorac Cardiovasc Surg 2010;58:184-5. [Crossref] [PubMed]
- Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin 2014;24:151-6. v.. [Crossref] [PubMed]
- Dunning J, Walker WS. Pulmonary artery bleeding caused during VATS lobectomy. Ann Cardiothorac Surg 2012;1:109-10. [PubMed]
- Yamashita S, Tokuishi K, Moroga T, et al. Totally thoracoscopic surgery and troubleshooting for bleeding in non-small cell lung cancer. Ann Thorac Surg 2013;95:994-9. [Crossref] [PubMed]
- Watanabe A, Koyanagi T, Nakashima S, et al. How to clamp the main pulmonary artery during video-assisted thoracoscopic surgery lobectomy. Eur J Cardiothorac Surg 2007;31:129-31. [Crossref] [PubMed]
- Kamiyoshihara M, Nagashima T, Ibe T, et al. A tip for controlling the main pulmonary artery during video-assisted thoracic major pulmonary resection: the outside-field vascular clamping technique. Interact Cardiovasc Thorac Surg 2010;11:693-5. [Crossref] [PubMed]
- Nakanishi R, Oka S, Odate S. Video-assisted thoracic surgery major pulmonary resection requiring control of the main pulmonary artery. Interact Cardiovasc Thorac Surg 2009;9:618-22. [Crossref] [PubMed]
- Nakanishi R, Yamashita T, Oka S. Initial experience of video-assisted thoracic surgery lobectomy with partial removal of the pulmonary artery. Interact Cardiovasc Thorac Surg 2008;7:996-1000. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg 1999;68:995-1001; discussion -2.
- Solli P, Spaggiari L, Grasso F, et al. Double prosthetic replacement of pulmonary artery and superior vena cava and sleeve lobectomy for lung cancer. Eur J Cardiothorac Surg 2001;20:1045-8. [Crossref] [PubMed]
- Venuta F, Ciccone AM. Reconstruction of the pulmonary artery. Semin Thorac Cardiovasc Surg 2006;18:104-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Surgical techniques and results for partial or circumferential sleeve resection of the pulmonary artery for patients with non-small cell lung cancer. Ann Thorac Surg 2007;83:1971-6; discussion 6-7.
- Ibrahim M, Maurizi G, Venuta F, et al. Reconstruction of the bronchus and pulmonary artery. Thorac Surg Clin 2013;23:337-47. [Crossref] [PubMed]
- Endo T, Tetsuka K, Yamamoto S, et al. Transection of left common pulmonary vein during left upper lobectomy: how should it be reconstructed? J Surg Case Rep 2012;2012.
- Nakamura T, Koide M, Nakamura H, et al. The common trunk of the left pulmonary vein injured incidentally during lung cancer surgery. Ann Thorac Surg 2009;87:954-5. [Crossref] [PubMed]
- Yu DP, Han Y, Zhao QY, et al. Pulmonary lobectomy combined with pulmonary arterioplasty by complete video-assisted thoracic surgery in patients with lung cancer. Asian Pac J Cancer Prev 2013;14:6061-4. [Crossref] [PubMed]
- Han Y, Zhou S, Yu D, et al. Video-assisted thoracic surgery (VATS) left upper sleeve lobectomy with partial pulmonary artery resection. J Thorac Dis 2013;5 Suppl 3:S301-3. [PubMed]
- Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000;24:27-30; discussion -1.
- Solaini L, Prusciano F, Bagioni P, et al. Long-term results of video-assisted thoracic surgery lobectomy for stage I non-small cell lung cancer: a single-centre study of 104 cases. Interact Cardiovasc Thorac Surg 2004;3:57-62. [Crossref] [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [Crossref] [PubMed]
- Roviaro G, Varoli F, Vergani C, et al. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest 2004;126:725-32. [Crossref] [PubMed]
- Ohtsuka T, Nomori H, Horio H, et al. Is major pulmonary resection by video-assisted thoracic surgery an adequate procedure in clinical stage I lung cancer? Chest 2004;125:1742-6. [Crossref] [PubMed]
- Shiraishi T, Shirakusa T, Hiratsuka M, et al. Video-assisted thoracoscopic surgery lobectomy for c-T1N0M0 primary lung cancer: its impact on locoregional control. Ann Thorac Surg 2006;82:1021-6. [Crossref] [PubMed]
- Jones RO, Casali G, Walker WS. Does failed video-assisted lobectomy for lung cancer prejudice immediate and long-term outcomes? Ann Thorac Surg 2008;86:235-9. [Crossref] [PubMed]
- Li Y, Wang J, Yang F, et al. Indications for conversion of thoracoscopic to open thoracotomy in video-assisted thoracoscopic lobectomy. ANZ J Surg 2012;82:245-50. [Crossref] [PubMed]
- Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55-61, 2 e1.
- Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011;35:590-5. [Crossref] [PubMed]
- Sawada S, Komori E, Yamashita M. Evaluation of video-assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg 2009;36:487-90. [Crossref] [PubMed]
Cite this article as: Safdie FM, Sanchez MV, Sarkaria IS. Prevention and management of intraoperative crisis in VATS and open chest surgery: how to avoid emergency conversion. J Vis Surg 2017;3:87.


