Salvage video-assisted thoracoscopic lobectomy for isolated local relapse after stereotactic body radiotherapy for early stage non-small cell lung cancer: technical aspects and perioperative management
Introduction
Stereotactic body radiotherapy (SBRT) has been increasingly recognized as an acceptable alternative treatment option to surgical resection for early-stage non-small cell lung cancer (NSCLC) in patients who are not ideal operative candidates (1-3).
Local relapse (LR) after SBRT for clinical stage I NSCLC is not uncommon and has been reported to be 4–11.9% at 3 years (4-6) and 10.5–28.3% at 5 years (3,4). Continuous efforts have been made to identify patients at higher risk of LR, while there is very few data upon management of isolated LR after SBRT. LR after SBRT for NSCLC is a dilemma since local treatments for this patient cohort are challenging: most of SBRT patients are not or marginally operable and repeating SBRT or offering conventional radiotherapy in the same area is concerning for safety and a degree of toxicity. Repeat SBRT for LR appears safe (6) and may achieve reasonable local control, although long-term survival outcomes remain unknown. Salvage surgery for isolated LR appears to be performed at low-risk operable patients (7-10), and we previously reported favorable long-term survival (11). In this article, we discuss salvage video-assisted thoracoscopic lobectomy for isolated LR in two patients.
Case presentation
In this article, we describe two patients of those who were found to have isolated LR during the follow-up after undergoing SBRT for a previously untreated, solitary, and peripheral clinical stage I (T1N0M0 or T2aN0M0) NSCLC and negative mediastinal lymph node staging with positron emission tomography-computed tomography (PET-CT) at Kyoto University Hospital between January 1999 and December 2013.
For SBRT, patients were immobilized with a stereotactic body frame (Elekta, Stockholm, Sweden). The internal target volume was determined considering computed tomography (CT) with a slow scan technique or 4D CT technique and tumor motion assessed by X-ray fluoroscopy. The planning target volume was defined as the internal target volume plus 5-mm margin. Irradiation was performed with 6-MV X-ray beams from a linear accelerator (Clinac 2300 C/D; Varian Medical Systems till April 2008: Novalis; BrainLab AG, Munich, Germany, thereafter) in multiple noncoplanar static ports.
LR was diagnosed primarily on the basis of enlargement of the local tumor on CT that continued for at least 6 months or on the basis of FDG-PET showing an intense uptake with a maximal standardized uptake value over 5 at 6 months.
Between 1999 and 2013, 308 patients underwent SBRT for clinical stage I NSCLC and of these, 49 patients were found to have isolated LR during at least 1 year of follow-up. Twelve patients underwent salvage surgery (11).
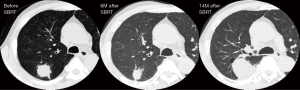
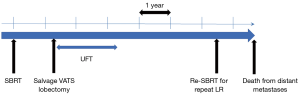
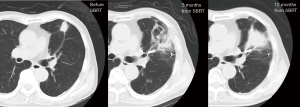
Patient A was a 75-year-old asymptomatic male presenting with a 2.8-cm solid pulmonary nodule in the right upper lobe. Transbronchial biopsy revealed it to be NSCLC on cytology. He had several comorbidities such as COPD (FEV1: 1.1 L), diabetes mellitus, angina pectoralis, previous history of smoking (66 pack-year), and ventricular arrhythmia (s/p catheter ablation for ventricular tachycardia). Although he was judged to be “operable” at presentation, he chose to undergo SBRT. The total radiation given was 4,800 cGy at the isocenter with a daily fractional dose of 1,200 cGy. A chest CT which was obtained 14 months after SBRT revealed an apparent tumor’s LR (Figure 1). The diameter of the tumor on CT was 5.0 cm. The patient was re-evaluated for a potential surgical resection and again judged to be operable at the multidisciplinary conference. Right upper lobectomy combined with right superior segment wedge resection and mediastinal lymph node dissection (LN #2R, #4R, #12) was performed via video-assisted thoracoscopic surgery (VATS) with two ports (including one access port in the 4th intercostal space). No intrapleural adhesion was noted (Figure 2) and the procedure was uncomplicated. The pericardial fat pad was sutured to the bronchial stump as coverage. The operative time was 3 h and 20 min. Due to prolonged air leak, the chest tube was kept in place for 10 postoperative days and the patient was discharged on the postoperative 16th day. The pathologic findings showed central necrosis and peripheral viable cells of squamous cell carcinoma (Figure 3), T2aN0M0 (stage IB). As a part of our practice, he was given oral uracil and tegafur as adjuvant chemotherapy for 2 years postoperatively. He had no sign of recurrence for 5 years and 2 months. However, on the follow-up CT, he was found to develop local recurrence at the resection margin of right lower lobe for which he underwent re-SBRT (4,800 cGy). Subsequently, he developed bone metastasis, multiple cerebral infarction and aspiration pneumonia and died at 6 years and 6 months postoperatively. The chronological sequence of these recurrences and treatments is shown in the Figure 4.
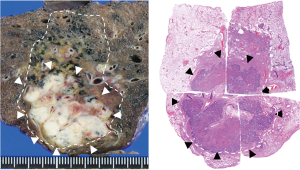
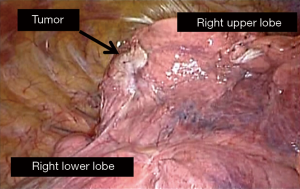
Patient B was an 84-year-old male presenting with a 3.5-cm mass in the left upper lobe. He was a previous smoker (45 pack-year). Transbronchial biopsy revealed it to be adenocarcinoma (epidermal tyrosine growth receptor: wild type, anaplastic lymphoma kinase genetic rearrangement: negative). He had no notable comorbidity. Although he was judged to be “operable”, he chose to proceed with SBRT. The total radiation given was 4,800 cGy at the isocenter with a daily fractional dose of 1,200 cGy. Chest CT 12 months after SBRT revealed an apparent consolidation, concerning for tumor relapse (Figure 5). The patient was re-evaluated for operability and judged as operable. Harvesting of an intercostal muscle flap, a left upper lobectomy and mediastinal lymph node dissection (LN#6, LN#10, and LN#11) was performed via VATS with three ports. Intraoperative findings included intrapleural adhesion on the mediastinal side. In addition, hilar lymph nodes were noted to adhere to the upper lobe bronchus as well as the interlobar pulmonary artery (Figure 6). The operative time was 5 h and 32 min and the bleeding amount was 200 mL. The postoperative course was complicated with prolonged and recurrent air leak requiring repeat chest tube drainage. He was discharged home on the 19th postoperative day. The pathologic examination showed moderately-differentiated adenocarcinoma, T2aN0M0, stage IB. Adjuvant chemotherapy was not performed, considering his age. Pathology confirmed sporadic viable cells and extensive necrosis. As of 2 years after the salvage surgery, the patient is doing well with no sign of recurrence.

Discussion
There is little known about long-term outcomes of salvage treatments for isolated LR after SBRT for early stage NSCLC. Besides, there is a dearth of data on what proportion of the operable patients with LR after SBRT for early-stage NSCLC underwent salvage surgery or repeat SBRT (7-10,13).
Clearly, operability is the required condition for salvage surgery for LR after SBRT. Unfortunately, operability is an unmeasurable factor difficult to define and presumably deriving from multidisciplinary conferences between radiation oncologists and thoracic surgeons. Also, the operability does not indicate the approach or the extent of pulmonary resection. We would recommend that operability be discussed multiple times, not only at SBRT but also at LR since operability is not always constant or fixed (10,13) as in our series (11). Most likely, patients with LR will require a lobectomy instead of sublobar resection as the extent of resection in terms of the tumor’s size and we should evaluate patients for potential salvage surgery from this viewpoint.
One of the technical factors that make surgeons hesitant to proceed with salvage surgery is potential intrapleural adhesions that are presumably derived from SBRT and may especially preclude a minimally invasive approach, among previous reports on salvage surgery after SBRT. Intrapleural adhesions at salvage surgery vary: there was no intrapleural adhesion in either case in Neri’s report (8), whereas adhesions were encountered in all patients of another series (9). Our previous series found intrapleural adhesions in 2 out of 12 patients and 5/12 (41.7%) of patients were operated on via VATS in contrast to the report of Allibhai et al. (9). Selection of a surgical approach in our series was typically related with surgeons’ experience and preference, and our findings suggest minimally invasive thoracic surgery, or VATS appears feasible at least in a portion of patients undergoing salvage surgery after SBRT. The VATS approach, generally, would be considered an advantage in perioperative short-term outcomes (14) especially in cardiopulmonary-compromised patients (15), as compared to open thoracotomy. On the other hand, as to patient A, prolonged pleural drainage was required postoperatively and patient B suffered from recurrent air leak requiring re-drainage. There is no scientific or statistical background for prolonged air leak in these patients, but we may need to anticipate a tendency of prolonged air leak following salvage lobectomy after SBRT. VATS approach appears feasible in patients undergoing salvage surgery, whereas no data exists as to the advantage of the approach in short-term or long-term outcomes.
Of note, patient A developed local recurrence 5 years after salvage surgery and received repeat SBRT of 4,800 cGy. Hearn et al. reported no grade 3 or higher toxicities after salvage (repeat) SBRT for local recurrence after initial SBRT in 22 patients (6). Gill et al. reported 13 patients undergoing salvage SBRT for local recurrence after sublobar resection for NSCLC (16). The short-term outcomes included grade 3 esophageal stricture and 8 patients (61.5%) developed a distant metastasis in the long term after salvage SBRT. Salvage SBRT after initial SBRT and its salvage lobectomy has not been reported to our knowledge. Salvage SBRT appears less invasive than a reoperative pulmonary resection, but we should await any upcoming data on outcomes of salvage SBRT.
In patient B, it was somewhat difficult to complete salvage VATS lobectomy in an 85-year-old male with intrapleural adhesions deriving from previous SBRT. We did encounter intrapleural adhesions on the mediastinal side and required careful dissections to protect the phrenic nerve and the recurrent laryngeal nerve. Also, we had some difficulty in finding a plane in the fissure and also in the hilar structure. Due to hilar lymphadenopathy, it takes time to dissect the upper lobe bronchus and we needed to divide the superior division bronchus and the lingular bronchus separately. In spite of the relatively long operative time, we did not convert to open thoracotomy or the procedure was not associated with perioperative morbidity, except for prolonged and recurrent air leak. Salvage VATS lobectomy may be worth trying even for an elderly patient at an experienced institution.
Lastly, this article presents more questions than answers, which should be discussed in the future. How long should patients be followed up? Is salvage VATS lobectomy associated with as low morbidity as upfront VATS lobectomy for early stage NSCLC? Which of salvage surgery and repeat SBRT is associated with more favorable outcomes in patients with isolated LR? The dearth of detailed data on patients with LR is a challenge in discussing those questions and, therefore, multidisciplinary conferences are essential.
Conclusions
We described two patients undergoing salvage VATS lobectomy for isolated LR after SBRT for early stage NSCLC. VATS approach appears feasible, but may require careful dissection of mediastinal adhesions deriving from SBRT. Multidisciplinary conferences are essential and should be continued for managing patients undergoing salvage surgery for LR after SBRT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Ishikura S. Optimal radiotherapy for non-small-cell lung cancer: current progress and future challenges. Gen Thorac Cardiovasc Surg 2012;60:127-31. [Crossref] [PubMed]
- Senan S, Paul MA, Lagerwaard FJ. Treatment of early-stage lung cancer detected by screening: surgery or stereotactic ablative radiotherapy? Lancet Oncol 2013;14:e270-4. [Crossref] [PubMed]
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [Crossref] [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Hearn JW, Videtic GM, Djemil T, et al. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys 2014;90:402-6. [Crossref] [PubMed]
- Chen F, Matsuo Y, Yoshizawa A, et al. Salvage lung resection for non-small cell lung cancer after stereotactic body radiotherapy in initially operable patients. J Thorac Oncol 2010;5:1999-2002. [Crossref] [PubMed]
- Neri S, Takahashi Y, Terashi T, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol 2010;5:2003-7. [Crossref] [PubMed]
- Allibhai Z, Cho BC, Taremi M, et al. Surgical salvage following stereotactic body radiotherapy for early-stage NSCLC. Eur Respir J 2012;39:1039-42. [Crossref] [PubMed]
- Taira N, Kawabata T, Ichi T, et al. Salvage operation for late recurrence after stereotactic body radiotherapy for lung cancer: two patients with no viable cancer cells. Ann Thorac Surg 2014;97:2167-71. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Treatment and Prognosis of Isolated Local Relapse after Stereotactic Body Radiotherapy for Clinical Stage I Non-Small-Cell Lung Cancer: Importance of Salvage Surgery. J Thorac Oncol 2015;10:1616-24. [Crossref] [PubMed]
- Hamaji M, Chen-Yoshikawa TF, Matsuo Y, et al. The operative procedure in patient B. Asvide 2017;4:255. Available online: http://www.asvide.com/articles/1564
- Robinson CG, DeWees TA, El Naqa IM, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:192-201. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28, dicussion 28-29.e1.
- Gill BS, Clump DA, Burton SA, et al. Salvage stereotactic body radiotherapy for locally recurrent non-small cell lung cancer after sublobar resection and i(125) vicryl mesh brachytherapy. Front Oncol 2015;5:109. [Crossref] [PubMed]
Cite this article as: Hamaji M, Chen-Yoshikawa TF, Matsuo Y, Motoyama H, Hijiya K, Menju T, Aoyama A, Sato T, Sonobe M, Date H. Salvage video-assisted thoracoscopic lobectomy for isolated local relapse after stereotactic body radiotherapy for early stage non-small cell lung cancer: technical aspects and perioperative management. J Vis Surg 2017;3:86.