VATS and open chest surgery in diagnosis and treatment of benign pleural diseases
Introduction
Pleura is the serous membrane that lines the thoracic cavity and surrounds the lungs. The word “pleura” is derived from the Greek word “πλευρά” which defines the side. Interestingly, the same word is used in Greek to describe not the serosa but the ribs of the thoracic cavity, while the word “υπεζωκότας” (i.e., underlayer) more appropriately defines the membrane.
This smooth tissue membrane consists of two layers of mesothelium, which secrete serous fluid. The inner layer that covers the surface of lungs, separating the different lobes, is called the “visceral pleura” while the outer layer, which is attached to the inner surface of the chest wall and separates the pleural cavity from the mediastinum, is called the “parietal pleura” (1).
The intrapleural pressure is defined as “negative” because it normally measures below the atmospheric pressure. This allows air to travel into the lungs during an inspiratory effort however it can also act as a vacuum for fluid, air and small particles from different parts of the body, allowing them to move into the pleural space and be retained in it, because of its ability to hold large amounts of liquid or air.
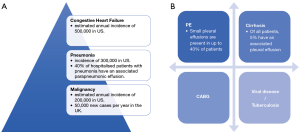
A pleural effusion is the result of fluid collection between the parietal and visceral pleural surfaces. Even though a thin layer of fluid is physiologically present in this space for purposes of lubrication and ease of movement of the lung during respiration, in pathophysiological circumstances the fluid can accumulate due to an imbalance of its normal flow, with either too much fluid produced or not enough removed. Pleural effusion can be a manifestation of many different conditions; the most common cause of pleural effusion in developed countries is congestive heart failure, followed by pneumonia and malignancy (2-6) (Figures 1-3).
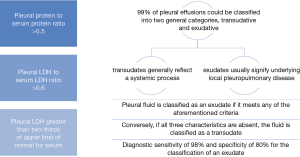
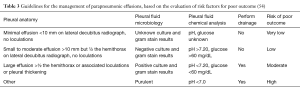
Prompt diagnosis and management of pleural effusions will offer the best outcome. A comprehensive medical history and careful physical examination can guide the clinician to correctly determine the nature of the effusion and distinguish between a transudate or exudate. The distinction between the two is not only an important first step towards obtaining a definitive diagnosis but can also direct additional investigations (9) (Table 1).
Further investigations should be undertaken with a chest radiograph and transthoracic ultrasound. In the event of complicated effusions with pleural thickness and/or loculations or when there’s suspicion of embolization or malignancy as the cause of the effusion, further imaging with contrast enhanced computed tomography should be obtained. A CT scan should always be performed prior to planned surgical management (9).
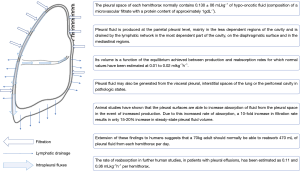
When possible, a diagnostic pleural aspiration should be performed. Image guidance may be considered, especially in more complex effusions, as it not only increases the rate of success but it can also reduce unintended complications. Samples from the fluid are then sent for further biochemistry, cytology and microbiology controls (9) (Figure 4).
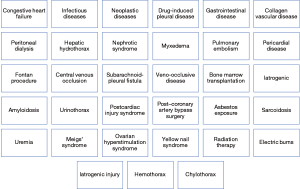
It is highly important that a systematic approach is undertaken during the investigation of pleural effusions to ensure that thorough checks have been performed which would lead to accurate diagnosis and appropriate treatment. Various algorithms have been proposed to guide clinicians navigate through the complex stages and evaluation and management (Figure 5), however, even after a complete investigation some patients will remain undiagnosed or diagnosed with “non-specific pleuritis”. Venekamp et al. contacted a retrospective study on 75 patients that had been previously diagnosed with “non-specific pleuritis” and found that 8.3% of them were eventually diagnosed with malignancy, over a 2-year follow-up period (14).
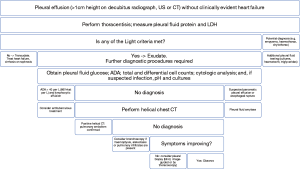
Treatment is based on the nature of the effusion and underlying condition while therapeutic thoracocentesis with nearly complete removal of the fluid collection alleviates symptoms in most circumstances. Patients unfit for invasive procedures (such as medical or surgical thoracoscopy) who remain undiagnosed, should stay under surveillance. A malignant process could be the underlying cause in a substantial number of undiagnosed effusions, thus routine follow-up is considered suitable.
Even though a small volume of pleural fluid can be found in the pleural space in physiological circumstances, the presence of air in the cavity is always due to pathophysiological causes. This abnormal air collection in the chest is called pneumothorax and can either be the result of direct trauma or due to diseased lung parenchyma.
Large amounts of liquid or air are not the only elements in the thoracic cavity that can lead to pleural disorders. Asbestos, as well as other inhaled toxic particles, can be retained into the pleural cavity resulting to diseases such as mesothelioma.
Asbestos is used to describe a group of naturally occurring fibrous minerals with exploitable physical properties such as strength, poor heat conduction and chemical resistance which had contributed to its widespread use in a variety of products, especially pipes and fittings (15). Asbestos exposure can most commonly manifest as benign pleural disease where pleural thickening may be encountered either in the form of well-defined parietal pleural plaques or as diffuse visceral pleural thickening. In many developed countries, the deleterious effects of asbestos exposure to human health are only now being observed and understood as a minimum lag time of ten years from the first asbestos exposure is required to attribute pleural disease to asbestos (16). Those previously exposed to asbestos, in professional or social settings, have an increased risk of being diagnosed with malignant pleural mesothelioma and lung cancer, while definitive diagnosis can often have shattering health and psychosocial impacts, not only to the ones immediately affected but also their families, relatives and broader social circles. Although the use of asbestos has now been banned in many industrialized countries, workers continue to be exposed to it due to repair and removal work while, contrary to recommendations, asbestos remains in use in newly developing countries. Vigorous research is currently ongoing to help better understand the characteristics and global burden of asbestos-related disease.
Surgical management of benign pleural diseases
Localised fibrous tumours of the pleura
Localised fibrous tumours are benign pleural lesions that have similar appearance to mesotheliomas and were believed in early years to originate from mesenchymal cells, however it was later found that they arise from the submesothelial mesenchymal layer (17). Asbestos exposure has been associated with the disease, and even though reports have disputed the correlation between localised fibrous tumours and previous exposure to carcinogens (18), the possibility of direct or indirect occupational contact in the past should be considered. Asbestos exposure with a low to moderate elevation of fibre concentration in the lungs was determined at a high percentage of these patients, as shown in a study by Kayser et al. (19).
Surgical resection represents the treatment of choice. A complete excision of the mass with clear resection margins and minimal resection of lung parenchyma should be performed. Extrapleural dissection as well as chest wall and anatomical lung resections should be undertaken as appropriate while redo surgery is highly recommended in the event of recurrence. Intraoperative frozen section should also be performed whenever possible, to ensure clear resection margins. Video-assisted thoracoscopic surgery (VATS) is the procedure of choice for the majority of these lesions while open surgery is reserved for larger masses (20-24). Localized benign localised fibrous tumours are almost always cured with complete surgical resection, however recurrences can occur even after many years have passed from the time of the initial resection (25).
Chylothorax
The accumulation of chylous fluid in the chest is called “chylothorax”. It can be the result of leakage from the lymphatic vessels, and most frequently from the thoracic duct. Underlying causes include trauma (blunt or penetrating) in the area between the upper abdomen and neck, cardiothoracic surgery and other diseases, such as lymphoma (26-28). Treatment options range from conservative to surgical, as well as newly developed interventional radiological procedures. However, due to the rare occurrence of the disease, there have been no prospective studies conducted and its management algorithm varies, fitting to the setting in which it develops.
Chylothorax can be difficult to differentiate from other pleural effusions. High-volume or rapidly occurring incidences can lead to dyspnoea, cough, chest pain, and hypovolaemia. Pleuritic pain and fever may be absent as chyle does not normally induce an inflammatory response (29). The clinical presentation is largely associated with nutritional deterioration, as a result of electrolyte, protein, lipid and vitamin depletion. Immunodeficiency and lymphopenia may also occur (30). Chylothorax should be considered in all patients with an appropriate history; thorough medical history taking is imperative.
Regardless of the approach, treatment should be started without delays; as many as half of patients left with untreated chylothorax are expected to die from ensuing complications (31,32). Generally, conservative treatment is initiated before more invasive measures are undertaken. The pleural cavity is drained from chyle by placement of an intercostal tube, while the patient follows a diet containing medium-chain triglycerides, otherwise total parenteral nutrition is commenced. Octreotide/somatostatin can be also added to reduce production of chyle (33). When conservative management fails, surgical treatment is indicated. The time when the patient will be taken back to theatres can depend on the volume and duration of drainage, response (or lack thereof) to conservative measures and clinical deterioration. As no data exist that would form a guideline, the timing is more often decided by individual surgeon’s preference, taking also into consideration the risks of reoperation when chylothorax has resulted as a complication of a previous procedure.
Ligation of the thoracic duct is the most common surgical approach with a success rate at around 95% (34). Identifying the structure or the leak is the most difficult part of the procedure and it can become easier by administering cream intraoperatively, via a nasogastric tube. If the leak cannot be visualised then mass ligation in the presumed course of the thoracic duct is indicated as it can often lead to successful control of the leak.
When chylothorax is presumed to be a post-operative complication, the decision regarding the side and type of the operation can be guided by the previous procedure or by drainage. In cases however when the origin of the leak is difficult to identify, special consideration is required before intervention, to avoid subjecting the patient to unnecessary procedures. A lymphangiogram can be requested prior to the operation, as it may detect the leak or even prove helpful in identifying abnormalities of the thoracic duct anatomy (the frequency of which can be as high as 40%). Lymphangiography can be difficult to perform and relies heavily on operator’s experience (35,36). For that reason, early surgical intervention is progressively chosen for treatment (37).
Surgical technique—thoracoscopic ligation of the thoracic duct
The patient is placed in lateral decubitus position with the operating table flexed to achieve optimal exposure of the posterior mediastinum. Adequate pain management can be reached with administration of bupivacaine 0.25% as intercostal blocks, positioning of an epidural catheter is not necessary. The thoracoscope and the utility incisions can be placed according to individual’s surgeon preference, depending on their desired approach and previous experience. A lower incision at the level of the diaphragmatic dome is often necessary as it can be used to retract the diaphragm inferiorly, allowing better visualisation. The inferior pulmonary ligament is divided to allow further mobilisation of the lung. The mediastinal pleura posterior to the hilum is then dissected to increase exposure to the posterior mediastinum. Depending on the side of the procedure the appropriate plane is developed, either between the azygos vein (on the right) or the descending aorta (on the left) and the neighbouring structures of the posterior mediastinum. Blunt exploration in the area will reveal the thoracic duct as a thin, tube-shaped, structure with occasional peristalsis. The duct is then dissected and clipped or stapled. If an adequate length of it is isolated, it can be excised and sent to histopathology for confirmation. Supra-diaphragmatic duct ligation has a success rate as high as 90% (38).
When the duct cannot be directly identified, mass ligation of fatty and lymphatic tissue is advised and fibrin glue can also be applied an adjunct to the ligation (39). Surgeons should be cautious not to deploy unnecessary clips as this can result in further injury and leak from a rather fragile duct (40). Pleurodesis may also be undertaken to diminish the chance of recurrence (41). Normally two large bore chest drains are positioned to ensure sufficient drainage and complete lung re-expansion. Postoperatively, patients follow a medium-chain triglyceride (MCT) diet, the duration of which may vary as per surgeons’ preferences. Timing of drain removal is guided by the amount of drainage and successful lung re-expansion on post-operative chest films. Pleurodesis in not indicated in patients with prolonged effusions and trapped lung.
Haemothorax
Most haemothoraces typically occur after trauma however they could also be the result of disorders such as pulmonary embolism, aortic aneurysm ruptures, malignant metastatic disease or haemostatic abnormalities (42).
VATS allows washout of the cavity and removal of clots while it can also help identify and control the bleeding source. Furthermore, it allows precise placement of chest tubes under vision.
In patients with complicated injuries and when excessive or persistent bleeding is encountered, open thoracotomy should be undertaken, as it allows for thorough exploration. In non-traumatic cases of haemothorax, which can be due to cavitary disease, necrotic lung tissue, arteriovenous malformations and vascular abnormalities, the underlying disease should be appropriately treated (43). Double lumen intubation may not be an option in case of emergency and the extend of the undertaking should be balanced on the risks and benefits. Following control of the bleeding, the surgeon might decide to plan for a subsequent procedure to correct the underlying disease, after the patient is stabilised.
Pneumothorax
Pneumothorax can cause partial or complete lung collapse while in extreme circumstances it can build up under such high pressure that it could stop blood returning to the heart, leading to circulatory failure and death, when it remains unrecognised and is not promptly treated (Table 2).
The diagnosis of pneumothorax is clinical at first instance, radiological confirmation should not delay treatment in the life-threatening conditions. Patients normally present with dyspnoea and/or pleuritic chest pain while small pneumothoraces may remain completely asymptomatic. Clinical examination findings include absence of tactile fremitus, reduced breath sounds and hyper-resonance on percussion.
Aetiology, size, symptoms and effects of the pneumothorax will guide its treatment. A small, primary spontaneous pneumothorax that is asymptomatic can be safely monitored with follow-up imaging while larger, symptomatic primary pneumothoraces normally respond well to treatment with needle aspiration or tube drainage. Traumatic and secondary pneumothoraces may be sufficiently managed only with tube thoracostomy. Surgical management options are retained for prevention of recurrence or when conventional treatment fails to control the air leak or fully re-expand the collapsed lung.
VATS pleurodesis is more favoured than open in the UK, even though a meta-analysis by Barker et al. showed a four-fold increase in recurrence rates with VATS compared to open (44). According to the British thoracic society guidelines, pleurectomy via an open thoracotomy has the lowest rate of recurrence (estimated approximately at 1%) in complex or recurrent pneumothoraces, while pleurectomy and pleural abrasion via VATS, even though better tolerated, has a recurrence rate estimated at around 5% (45).
The principle in surgical management of pneumothorax remains unchanged since 1941 when Tyson and Crandall (46) described open thoracotomy for pleural abrasion, and 1956 when Gaensler (47) introduced parietal pleurectomy for recurrent pneumothoraces: surgery aims to facilitate formation of adhesions between the lung and chest wall preventing air from accumulating in the pleural space, thus resulting to lung collapse. Apicectomy or bullectomy is also undertaken to minimise the rate of recurrence, by removing the most diseased parts of the lung parenchyma. Pleurodesis can be achieved also by chemical means with the use of sclerosing agents such as tetracycline derivatives, bleomycin, talc and others.
Most surgeons perform minimally invasive procedures such as VATS or a transaxillary mini-thoracotomy for apical pleurectomy or abrasion. VATS is believed to offer advantages in pain management, shorter postoperative hospital stays and lesser costs, but no trial has offered definitive confirmation yet (45).
Pleural sepsis
Pleural infection is a serious clinical condition which affects approximately 65,000 patients every year in the UK and can result in mortality rates as high as 20% of reported cases. Of those who suffer with empyema, up to 40% may require surgical intervention for decontamination of the pleural space, and their hospital stay can extend to many weeks or months before the disease is under control (48).
Pleural effusion has been a challenge for physicians since it was first described by Imhotep in ancient Egypt at around 3000 BC. During the 19th century, pleural effusion was managed primarily via open thoracic drainage, until 1876, when closed tube drainage was first described. The influenza epidemic which occurred between 1917–1919 resulted in wider adoption of this technique. Introduction of antibiotics in the decade of the 1940s reduced the incidence of empyema and altered its bacteriology. Fibrinolytic therapy was first suggested in 1949. VATS techniques have been introduced only recently (49).
The selection of treatment as well as timing of intervention remains a debatable issue until today, among pulmonologists and thoracic surgeons (50). The fact that the overall incidence of pleural infection is increasing (51) in combination with data indicating that it mostly occurs in children and the elderly (52), raises the significance of establishing accurate diagnostic and treatment guidelines that will deliver timely and efficient results (49). Delay in initiating effective drainage may lead to increased mortality and morbidity (53).
Inflammation because of a respiratory tract infection, or due to either trauma or previous surgical chest intervention, can lead to fluid accumulation in the pleural cavity. The fluid may be sterile, but can progress to an empyema if left untreated. The development of parapneumonic effusions to an empyema has been described to follow three stages, over a period of 3–6 weeks. Stage 1 is defined as the exudative stage, stage 2 is the fibrinopurulent and loculated stage, while stage 3 is the chronic, organising, cortical stage. Lung entrapment and restriction of movement of the chest wall may result as empyema progresses. Eventually a thick fibrinous layer forms, encasing the lung and chest wall, creating a fibrothorax. Necrosis, formation of fistulae, as well as local and distant spread of the infection may also ensue (Table 3).
Full table
Decision making in the management of empyema is complex and not well standardised. Choice of treatment is based on the aetiology and the stage of the disease, taking into consideration the patient’s general condition. The basic management principles include systemic treatment of the underlying cause of infection and removal of infected fluid and tissue from the chest cavity. Surgical debridement can facilitate lung re-expansion at advanced stages of the disease while care should be taken to implement supportive measures that will improve the patient’s condition, thus preventing further complications or recurrence.
Not all patients will be managed efficiently by therapeutic thoracentesis or tube thoracostomy, however these techniques may be implemented before undertaking a more definitive drainage procedure. Fibrinolytics (33), VATS and open surgery are all acceptable approaches for managing patients with large and complicated effusions as they offer low mortality rates and decreased need for re-intervention (33).
In early stage empyema, chest tube drainage, antibiotics and fibrinolytic drugs are recommended; failure requires surgical intervention. Fibrinolysis should be the first-line therapy for children with empyema as it poses reduced risk of acute clinical deterioration. VATS plays a significant therapeutic role in the fibrinopurulent stage of empyema, in which loculated fluid cannot be adequately drained by chest tubes and fibrinolytic drugs alone. VATS also has an increasing effect in the treatment of organising phase empyema.
Open decortication is the standard treatment for patients with massive trapped lung. Open surgical procedures (decortication, window thoracostomy, thoracomyoplasty) are often used secondarily, to treat a recurring or an unresolved empyema. Vacuum-assisted closure (VAC) therapy with open window thoracostomy should be considered as soon as possible, in complicated or complex pleural empyema (e.g., post-pneumonectomy empyema) (55) (Figure 6).
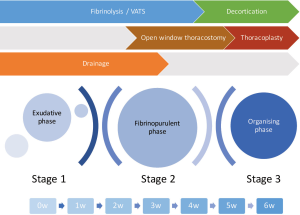
Surgical intervention aims to control sepsis, by facilitating removal of necrotic tissue from the chest cavity (debridement), and obliterate the empyema cavity, by allowing the trapped lung to re-expand via peeling of the organised cortex from its visceral pleura (decortication). Parietal pleurectomy can be further undertaken to completely eradicate the empyematic cavity (empyemectomy), free the diaphragm from thickened pleura and reduce the restrictive syndrome by preventing formation of fibrothorax (57). Diagnostic bronchoscopy should precede any surgical intervention to exclude presence of endobronchial obstruction that would prevent lung re-expansion.
VATS
The use of VATS in postpneumonic pleural infection is progressively expanding not just as an alternative to traditional open thoracotomy but also as an alternative option in earlier stages of empyema (58). It is not uncommon for patients with parapneumonic effusions to have impaired coagulation or other co-morbidities that would deem them as high risk for extensive open decortications and prolonged general anaesthesia (59). VATS can be offered to these patients as a more conservative tool, significantly decreasing operating times and bleeding, while applying lower impact on post-op ventilator mechanics of the chest wall and the diaphragm. Even in early stages of empyema VATS has a role as a more aggressive and decisive intervention, because it allows visualisation of the entire thoracic cavity which can result to timely, effective debridement (60). VATS technique has further evolved from being used as a “staging procedure” for diagnostic purposes to an interventional one, even in later stages of empyema (61).
VATS vs. conservative therapy for fibrinopurulent phase or complicated PPE or light III-V
Wozniak et al. (60) in their study “Choice of first intervention is related to outcomes in the management of empyema” concluded that a strategy of early VATS or thoracotomy is associated with better outcomes, in advanced empyema. Another study by Luh et al. (33) proved that VATS is safe and effective for treatment of complicated parapneumonic effusions and pleural empyema, while earlier intervention with VATS can produce better clinical results. A randomised trial by Wait et al. (62) demonstrated that VATS as a primary strategy is more effective in patients with complex and loculated empyema, and can result in shorter hospital stay and reduced costs, compared to fibrinolytic therapy. Shorter hospital stay and reduced need of open decortication after VATS was also demonstrated in a study by Bilgin et al. (63).
VATS vs. conservative therapy for organizing phase or empyema or light VI-VIII
As early as in 1996, VATS debridement had been compared to open thoracotomy (61) and was shown to have the same rate of success, while offering considerable benefits in hospital stay and cosmesis. Tong at al. (64) looked into VATS versus open debridement for benign conditions between 1996 to 2006 and found significantly fewer postoperative complications in the VATS group in the categories of atelectasis, prolonged air leak, reintubation, ventilator dependence, need for tracheostomy, blood transfusion, sepsis, and 30-day mortality. They concluded that thoracoscopic decortication is effective and can be considered as a first option in most patients with complex pleural effusions and empyema, as it can provide results which are comparable to those achieved by an open decortication, if not better.
Surgical technique
Following induction to general anaesthesia and double lumen intubation, the chest cavity is accessed via 1-, 2- or 3-port VATS, following lung isolation. An initial 2-cm incision below the tip of the scapula allows digital exploration to free adhesions before the thoracoscope is advanced. Suction alone might be enough to manage a simple, uncomplicated parapneumonic effusion at an early stage.
When further access to the thoracic cavity is needed, the camera is used to visualise optimal access areas for the extra ports. This prevents causing injury to the lung parenchyma if it’s adhered to the chest wall and avoids damaging the neurovascular bundle. A large-bore chest drain connected to suction and attached to long-handled artery forceps may be used to allow breaking down and draining the multiseptated collections. After the chest cavity is thoroughly debrided and the lung is decorticated as necessary, the anaesthetist is asked to re-inflate the lung, to determine under direct vision any need for further decortication. To develop a correct plane, the surgeon needs to sharply incise the visceral cortex and peel off the thick layer from the surface of the lung, with the help of a mounted pledget. Continuous positive pressure ventilation may further facilitate this process. Post-operative air leaks can be reduced by employing surgical sealants. The VATS technique can provide the desirable drainage outcomes while causing less trauma to the healthy tissues and inflicting less pain, thus resulting to reduced hospital stay (65). It’s important to note that the success of VATS decortication can be dependent on operative experience as well as the timing of referral, as delayed involvement of surgeons can increase the need for conversion to open thoracotomy.
Randomised control trials should be designed to evaluate the appropriate timing of surgical intervention in management of parapneumonic effusions, as the quality of evidence derived from the existing studies is widely affected by methodological limitations and cannot produce objective criteria that may result to strong recommendations.
Open Techniques
Zahid et al. (66) investigated into the best treatment option for management of post-pneumonectomy empyema and concluded that open procedures were associated with reduced rate of recurrence, rate of re-intervention and mortality, when compared to minimally invasive approaches. Despite being used in possibly more complicated clinical settings, open surgery had a high rate of success and was associated with reduced hospital stay and need for re-intervention.
Open thoracotomy
Open thoracotomy decortication is the most invasive surgical option for management of empyema and remains the gold standard approach for getting the trapped lung to re-expand. The pleural abscess cavity is thoroughly excised and removed completely during this procedure. Access to the thoracic cavity is achieved through a posterolateral thoracotomy at the level of the 5th or 6th intercostal space. The surgeon initially performs parietal decortication until normal lung parenchyma is identified and then proceeds by further developing the surgical plane between the wall of the abscess cavity and the visceral pleura. Correct timing of the operation is key to its success as it should fall after the organising phase of the empyema without however being delayed to the point of onset of a fixed fibrothorax. Lung decortication is expected to restore its mechanical function, as well as improve its impaired vital capacity (67).
Open window thoracostomy +/- partial rib resection
Leo Eloesser was the first to describe a procedure for creating a flap thoracostomy window in 1935 (68). The procedure was later modified by Symbas and coworkers (69) and had been used as a treatment option for patients diagnosed with tuberculosis as well as pleural space infections which had been associated with bronchopleural fistulae. Alternative adaptations of the thoracostomy window, such as the Clagett window (70), have also been described.
Even though this procedure is not routinely performed anymore in most thoracic centres, it can still be used for patients presenting with complex empyema and especially for those that have undergone previous pneumonectomy procedures and then present with an infected post-pneumonectomy space. It may also be indicated for patients who are medically unstable (i.e., intubated and mechanically ventilated in intensive care) or not fit enough to tolerate a surgical decortication. Its main advantage is that it allows anatomical access to the thoracic cavity, that facilitates drainage of pus. Rib resection makes it possible to maintain a permanent drainage route, a practice that is further aided by positioning of corrugated drains through the window directly in the cavity. A specific advantage of the thoracostomy window procedure is that it serves as an intermediate step for sterilising the infected cavity before planning for further preventive or treatment interventions, such as closure of bronchopleural fistulae (55).
This technique can also be performed under local anaesthetic for patients that are unfit to undergo general anaesthesia. A large-bore intercostal tube is positioned in the infected cavity for purposes of drainage (71). This “empyema tube” is cut at the wound edge and is connected to a stoma bag for collection of fluid. It’s important that a hole is made in the bag to release air, when an air leak is present. The tube is gradually withdrawn at weekly intervals as the cavity closes, a process that can normally take up to some months. Antibiotics can also be instilled in the cavity before final closure.
The Eloesser technique continues to evolve with the advancement of modern treatment options (72). Several studies are currently reporting good results from augmentation of the thoracostomy window with a VAC device (73,74).
Space-filling procedures
These procedures are reserved for the rare occasions of persistent and recurrent infections of the pleural cavity, as for example in the event of post-pneumonectomy space infections. The aim is to eliminate the space between the lung and the chest wall and this can be achieved by performing either a thoracoplasty, which causes the cavity to collapse, or a thoracomyoplasty, which involves using viable muscle tissue to fill up the cavity. Thoracoplasty is indicated when conservative measures have not proven sufficient or successful and requires prior control of the infection. A bronchopleural fistula may or may not be present in the residual pleural space (75).
The thoracoplasty procedure is normally performed via a posterolateral incision which is extended to expose the upper ribs. To achieve satisfactory collapse of the cavity, enough ribs might need to be resected, while it should be avoided entering the pleural cavity. Adequate mobilisation of the soft tissues can achieve vertical collapse and approximate them to the mediastinum. This might involve apicolysis, resection of the transverse processes, disarticulation of posterior aspects of the ribs and scapular resections to achieve desirable results. An intrathoracic muscular transposition can finally be performed to totally obliterate the cavity in one stage (75).
Conclusions
Pleural disease can be a result of a wide variety of pathologies, thus a systematic approach to diagnosis and management should be applied. Light’s criteria can often help differentiate between a systemic disease, which manifests as a transudative effusion, from local disease which leads to an exudative effusion.
Prompt diagnosis cannot always be made, as the onset of symptoms can be gradual or masked and patients may present in advanced stages of their disease, when conservative treatment measures may not be able to provide a desirable outcome.
Surgical thoracoscopy may be employed for visual assessment of the pleural space and sampling of tissue, while it may also be used for management of complicated collections. Open thoracotomy remains the gold standard, however with the advancement of thoracoscopic instruments and techniques, minimally invasive approaches now provide comparable outcomes and have been taking over the management of benign pleural diseases.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Light RW. Pleural Diseases. Baltimore: Lippincott Williams & Wilkins, 2007:(1).
- Light RW. Pleural Effusion. New England Journal of Medicine 2002;346:1971-7. [Crossref] [PubMed]
- Light RW. Parapneumonic Effusions and Empyema. Proceedings of the American Thoracic Society 2006;3:75-80. [Crossref] [PubMed]
- Light RW. The undiagnosed pleural effusion. Clin Chest Med 2006;27:309-19. [Crossref] [PubMed]
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001;7:198-201. [Crossref] [PubMed]
- Light RW, Rogers JT, Moyers JP, et al. Prevalence and clinical course of pleural effusions at 30 days after coronary artery and cardiac surgery. Am J Respir Crit Care Med 2002;166:1567-71. [Crossref] [PubMed]
- Brunelli A, Beretta E, Cassivi SD, et al. Consensus definitions to promote an evidence-based approach to management of the pleural space. A collaborative proposal by ESTS, AATS, STS, and GTSC. Eur J Cardiothorac Surg 2011;40:291-7. [Crossref] [PubMed]
- Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 1997;10:219-25. [Crossref] [PubMed]
- Hooper C, Lee YCG, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii4-ii17. [Crossref] [PubMed]
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009;301:309-17. [Crossref] [PubMed]
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13. [Crossref] [PubMed]
- Porcel JM, Peña JM, Vicente de Vera C, et al. Reappraisal of the standard method (Light's criteria) for identifying pleural exudates. Med Clin (Barc) 2006;126:211-3. [Crossref] [PubMed]
- Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician 2006;73:1211-20. [PubMed]
- Venekamp LN, Velkeniers B, Noppen M. Does 'idiopathic pleuritis' exist? Natural history of non-specific pleuritis diagnosed after thoracoscopy. Respiration 2005;72:74-8. [Crossref] [PubMed]
- Dodson RF, Hammar SP. Asbestos. CRC Press, 2011:(1).
- Wolff H, Vehmas T, Oksa P, et al. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health 2015;41:5-15. [Crossref] [PubMed]
- Chan JK. Solitary fibrous tumour--everywhere, and a diagnosis in vogue. Histopathology 1997;31:568-76. [Crossref] [PubMed]
- Suter M. Localized fibrous tumours of the pleura: 15 new cases and review of the literature. European Journal of Cardio-Thoracic Surgery 1998;14:453-9. [Crossref] [PubMed]
- Kayser K, Trott J, Böhm G, et al. Localized fibrous tumors (LFTs) of the pleura: clinical data, asbestos burden, and syntactic structure analysis applied to newly defined angiogenic/growth-regulatory effectors. Pathol Res Pract 2005;201:791-801. [Crossref] [PubMed]
- Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control 2006;13:264-9. [PubMed]
- Liu J, Cai C, Wang D, et al. Video-assisted thoracoscopic surgery (VATS) for patients with solitary fibrous tumors of the pleura. J Thorac Oncol 2010;5:240-3. [Crossref] [PubMed]
- Cardillo G, Carbone L, Carleo F, et al. Solitary Fibrous Tumors of the Pleura: An Analysis of 110 Patients Treated in a Single Institution. The Annals of Thoracic Surgery 2009;88:1632-7. [Crossref] [PubMed]
- Takahama M, Kushibe K, Kawaguchi T, et al. Video-Assisted Thoracoscopic Surgery Is a Promising Treatment for Solitary Fibrous Tumor of the Pleura. Chest 2004;125:1144-7. [Crossref] [PubMed]
- Kohler M, Clarenbach CF, Kestenholz P, et al. Diagnosis, treatment and long-term outcome of solitary fibrous tumours of the pleura. European Journal of Cardio-Thoracic Surgery 2007;32:403-8. [Crossref] [PubMed]
- Okike N, Bernatz PE, Woolner LB. Localized mesothelioma of the pleura: benign and malignant variants. J Thorac Cardiovasc Surg 1978;75:363-72. [PubMed]
- Romero S. Nontraumatic chylothorax. Current Opinion in Pulmonary Medicine 2000;6:287-91. [Crossref] [PubMed]
- Fahimi H, Casselman FP, Mariani MA, et al. Current management of postoperative chylothorax. The Annals of Thoracic Surgery 2001;71:448-50. [Crossref] [PubMed]
- O'Callaghan AM, Mead GM. Chylothorax in lymphoma: mechanisms and management. Ann Oncol 1995;6:603-7. [Crossref] [PubMed]
- Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. European Journal of Cardio-Thoracic Surgery 2007;32:362-9. [Crossref] [PubMed]
- Marts BC, Naunheim KS, Fiore AC, et al. Conservative versus surgical management of chylothorax. Am J Surg 1992;164:532-4; discussion 534-5. [Crossref] [PubMed]
- Lagarde SM, Omloo JM, de Jong K, et al. Incidence and management of chyle leakage after esophagectomy. Ann Thorac Surg 2005;80:449-54. [Crossref] [PubMed]
- Hölscher AH, Vallböhmer D, Brabender J. The prevention and management of perioperative complications. Best Pract Res Clin Gastroenterol 2006;20:907-23. [Crossref] [PubMed]
- Luh SP, Chou MC, Wang LS, et al. Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest 2005;127:1427-32. [PubMed]
- Schild HH, Strassburg CP, Welz A, et al. Treatment options in patients with chylothorax. Dtsch Arztebl Int 2013;110:819-26. [PubMed]
- Cerfolio RJ, Allen MS, Deschamps C, et al. Postoperative chylothorax. J Thorac Cardiovasc Surg 1996;112:1361-5; discussion 1365-6. [Crossref] [PubMed]
- Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol 2011;28:63-74. [Crossref] [PubMed]
- Pillay TG, Singh B. A review of traumatic chylothorax. Injury 2016;47:545-50. [Crossref] [PubMed]
- Lee KH, Jung JS, Cho SB, et al. Thoracic Duct Embolization with Lipiodol for Chylothorax due to Thoracic Endovascular Aortic Repair with Debranching Procedure. The Korean Journal of Thoracic and Cardiovascular Surgery 2015;48:74-7. [Crossref] [PubMed]
- Misthos P, Kanakis MA, Lioulias AG. Chylothorax complicating thoracic surgery: conservative or early surgical management? Updates Surg 2012;64:5-11. [Crossref] [PubMed]
- Ahmed SU, Sancheti MS, Pickens A. Thoracoscopic Thoracic Duct Ligation. Operative Techniques in Thoracic and Cardiovascular Surgery 2012;17:292-301. [Crossref]
- Sahn SA. Management of malignant pleural effusions. Monaldi Arch Chest Dis 2001;56:394-9. [PubMed]
- Gallardo X, Castañer E, Mata JM. Benign pleural diseases. Eur J Radiol 2000;34:87-97. [Crossref] [PubMed]
- Richardson JD, Miller FB, Carrillo EH, et al. Complex thoracic injuries. Surg Clin North Am 1996;76:725-48. [Crossref] [PubMed]
- Barker A, Maratos EC, Edmonds L, et al. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomised and non-randomised trials. Lancet 2007;370:329-35. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Tyson MD, Crandall WB. The surgical treatment of recurrent idiopathic spontaneous pneumothorax. J Thorac Surg 1941;10:566-70.
- Gaensler EA. Parietal pleurectomy for recurrent spontaneous pneumothorax. Surg Gynecol Obstet 1956;102:293-308. [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled Trial of Intrapleural Streptokinase for Pleural Infection. N Engl J Med 2005;352:865-74. [Crossref] [PubMed]
- Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii41-53. [Crossref] [PubMed]
- Rosenstengel A. Pleural infection-current diagnosis and management. J Thorac Dis 2012;4:186-93. [PubMed]
- Farjah F, Symons RG, Krishnadasan B, et al. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg 2007;133:346-51. [Crossref] [PubMed]
- Finley C, Clifton J, FitzGerald JM, et al. Empyema: An Increasing Concern in Canada. Canadian Respiratory Journal 2008;15:85-9. [Crossref] [PubMed]
- Chu MW, Dewar LR, Burgess JJ, et al. Empyema thoracis: lack of awareness results in a prolonged clinical course. Can J Surg 2001;44:284-8. [PubMed]
- Colice GL, Curtis A, Deslauriers J, et al. Medical and Surgical Treatment of Parapneumonic Effusions. Chest 2000;118:1158-71. [Crossref] [PubMed]
- Hofmann H-S. Modern Management of Empyema Thoracis. Seminars in Thoracic and Cardiovascular Surgery 2013;25:287-91. [Crossref] [PubMed]
- Molnar TF. Current surgical treatment of thoracic empyema in adults. Eur J Cardiothorac Surg 2007;32:422-30. [Crossref] [PubMed]
- Sensakovic WF, Armato SG, Starkey A, et al. Quantitative measurement of lung reexpansion in malignant pleural mesothelioma patients undergoing pleurectomy/decortication. Acad Radiol 2011;18:294-8. [Crossref] [PubMed]
- Waller DA. Thoracoscopy in management of postpneumonic pleural infections. Curr Opin Pulm Med 2002;8:323-6. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Fabbi E, et al. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg 2010;37:594-601. [Crossref] [PubMed]
- Wozniak CJ, Paull DE, Moezzi JE, et al. Choice of first intervention is related to outcomes in the management of empyema. Ann Thorac Surg 2009;87:1525-30; discussion 1530-1. [Crossref] [PubMed]
- Angelillo Mackinlay TA, Lyons GA, Chimondeguy DJ, et al. VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema. Ann Thorac Surg 1996;61:1626-30. [Crossref] [PubMed]
- Wait MA, Sharma S, Hohn J, et al. A randomized trial of empyema therapy. Chest 1997;111:1548-51. [Crossref] [PubMed]
- Bilgin M, Akcali Y, Oguzkaya F. Benefits of early aggressive management of empyema thoracis. ANZ J Surg 2006;76:120-2. [Crossref] [PubMed]
- Tong BC, Hanna J, Toloza EM, et al. Outcomes of video-assisted thoracoscopic decortication. Ann Thorac Surg 2010;89:220-5. [Crossref] [PubMed]
- Waller DA, Rengarajan A. Thoracoscopic decortication: a role for video-assisted surgery in chronic postpneumonic pleural empyema. Ann Thorac Surg 2001;71:1813-6. [Crossref] [PubMed]
- Zahid I, Routledge T, Billé A, et al. What is the best treatment of postpneumonectomy empyema? Interact Cardiovasc Thorac Surg 2011;12:260-4. [Crossref] [PubMed]
- Bölükbas S, Eberlein M, Schirren J. Prospective study on functional results after lung-sparing radical pleurectomy in the management of malignant pleural mesothelioma. J Thorac Oncol 2012;7:900-5. [Crossref] [PubMed]
- Eloesser L. An Operation For Tuberculous Empyema. Diseases of the Chest 1935;1:8-23. [Crossref]
- Symbas PN, Nugent JT, Abbott OA, et al. Nontuberculous Pleural Empyema in Adults. The Annals of Thoracic Surgery 1971;12:69-78. [Crossref] [PubMed]
- Clagett OT, Geraci JE. A procedure for the management of postpneumonectomy empyema. J Thorac Cardiovasc Surg 1963;45:141-5. [PubMed]
- Biswas A, Jantz MA, Penley AM, et al. Management of chronic empyema with unexpandable lung in poor surgical risk patients using an empyema tube. Lung India 2016;33:267-71. [Crossref] [PubMed]
- Thourani VH, Lancaster RT, Mansour KA, et al. Twenty-six years of experience with the modified eloesser flap. Ann Thorac Surg 2003;76:401-5; discussion 405-6. [Crossref] [PubMed]
- Varker KA, Ng T. Management of empyema cavity with the vacuum-assisted closure device. Ann Thorac Surg 2006;81:723-5. [Crossref] [PubMed]
- Palmen M, Van Breugel HN, Geskes GG, et al. Open window thoracostomy treatment of empyema is accelerated by vacuum-assisted closure. Ann Thorac Surg 2009;88:1131-6. [Crossref] [PubMed]
- Stefani A, Jouni R, Alifano M, et al. Thoracoplasty in the current practice of thoracic surgery: a single-institution 10-year experience. Ann Thorac Surg 2011;91:263-8. [Crossref] [PubMed]
Cite this article as: Perikleous P, Rathinam S, Waller DA. VATS and open chest surgery in diagnosis and treatment of benign pleural diseases. J Vis Surg 2017;3:84.