Planning video-assisted thoracic surgery segmentectomy using three dimensional computed tomography angiography and bronchography with a virtual safety margin
Introduction
The use of video-assisted thoracic surgery (VATS) segmentectomy for early-stage lung cancer, including solid nodules and ground glass nodules (GGNs), has been increasing because the technique preserves pulmonary function better than lobectomy. Compared with open surgery, the VATS approach has a number of benefits in the immediate postoperative period, including fewer complications and a shorter length of hospital stay (1). However, VATS requires surgeons to view the operative field on a 2-dimensional video monitor, and because they cannot palpate the organs directly, this frequently creates difficulties in judging anatomic relations (2). It is important to identify the intersegmental pulmonary veins that divide the pulmonary segments, especially when performing segmentectomy. Therefore, preoperative anatomic imaging is important.
Many studies have reported the efficacy of 3-dimensional computed tomography angiography and bronchography (3D-CTAB) for preoperative assessment of the pulmonary vessels and bronchi (3-7). However, it is difficult for 3D-CTAB alone to recognize the recommended safety margins, which are currently 2 cm from the primary tumor. Therefore, we adopted a scheme that merges a virtual 3D safety margin with 3D-CTAB for patients with lung cancer (8,9). We report two patients with double primary lung cancer who underwent segmentectomy based on preoperative simulation 3D-CTAB with a virtual 3D safety margin.
Contrast enhanced CT
In our institution, 3D-CTAB data are obtained using a high-speed 64-channel or 128-channel multi-detector CT scanner (Aquilion 64, Toshiba Medical Systems, Tokyo, Japan; SOMATOM Definition Flash, Siemens Japan, Tokyo, Japan). Vascular access was obtained using a 20-gauge needle in the right median cubital vein. For vessel enhancement, 96 mL of a nonionic contrast medium was used at a flow rate of 3.0 to 4.0 mL/s using a power injector (8,10). The injection of the contrast medium was immediately followed by 24 mL of saline at the same flow rate. To scan during the suitable arterial phase, the scan delay was decided using an automatic bolus-tracking system. Axial thin-section CT images of the whole lung were reconstructed using a slice thickness of 1 mm, a slice increment of 0.8–1 mm, and a standard algorithm.
3D-CTAB reconstruction
The thin-section CT images were transferred to a commercial 3D workstation (Ziostation, Amin Co. Ltd., Tokyo, Japan; Synapse Vincent, Fujifilm Medical, Tokyo, Japan), where a chest radiologist constructed the thoracic 3D-CTAB. The lung, primary tumor, pulmonary vessels, and the trachea and bronchi were separately segmented and color-coded by CT value, as defined by the tissue’s Hounsfield units (Figures 1,2) (8,11).
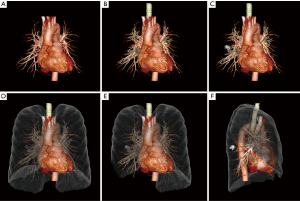

Virtual 3D safety margin
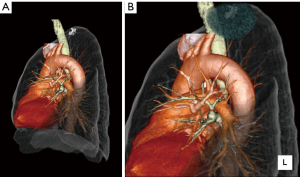
The safety margin was defined as a sphere, extending 2 cm outside the primary tumor. For example, the margin was 5 cm in diameter for a primary tumor of 1 cm, and the margin was 6 cm in diameter for a primary tumor of 2 cm (8). We typically drew the sphere in blue (Figures 3,4) (12). The center of the virtual 3D safety margin was manually matched to the center of the tumor using the 3D workstation. The radiologists and surgeons interpreted the relation between the margin and the intersegmental veins. In cases where the intersegmental veins ran through the safety margin, the veins were designated for removal.

Case presentation
Patient 1
A man in his 60s was referred to our hospital because of suspicion for a double primary lung cancer in the right lung. Examination with CT showed a pure GGN measuring 12 mm between right S4 and S5, and a single nodule measuring 15 mm in right S6 (Figures 5A-C,6) (13). As the patient had comorbid chronic obstructive pulmonary disease [forced expiratory volume in 1 second (FEV1) =1.76 L; FEV1 as a percentage of forced vital capacity =58.5%], we anticipated that bilobectomy of the right middle and lower lobe would be difficult. Therefore, we planned a right middle lobectomy and S6 segmentectomy based on the results of 3D-CTAB with a virtual 3D safety margin.
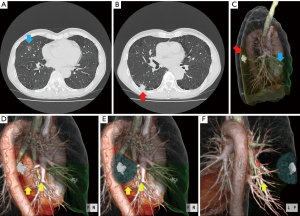

Figures 5D and 6 show that the results of 3D-CTAB were difficult to interpret in the right lower lobe, and for the distance between the tumor and the intersegmental vein V6c (between S6 and the basal segment). Figure 5E,F and Figure 7 (14) show that the course of V6c was outside the virtual 3D safety margin, which was 5.5 cm in diameter. Therefore, in a preoperative conference with respiratory medicine, thoracic surgery, and radiology, we deemed it possible to secure a sufficient surgical margin with right S6 segmentectomy. After lobectomy of right middle lobe, the pulmonary artery and vein to the involved segment (A6, V6) were resected. Next, the right lower lobe was inflated and segmental bronchus (B6) was divided. After collapsed S6, the surgeons confirmed inflation-deflation line and removed S6 using stapling device. Pathologic findings confirmed the diagnosis of well-differentiated adenocarcinoma in the middle lobe and moderately differentiated squamous cell carcinoma in S6; the surgical margins were free of tumor. No recurrence was seen at last follow-up, 5 years after surgery.

Patient 2
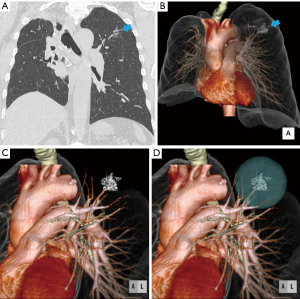
A man in his 70s was referred to our hospital because of a high suspicion for primary lung cancer in the left lung. The results of CT showed a pure GGN measuring 20 mm in left S1+2 (Figure 8A,B). The patient had a history of a right upper lobectomy for large cell lung carcinoma 8 years prior. Therefore, we planned a left upper-division segmentectomy based on the results of 3D-CTAB with a virtual 3D safety margin. Figure 8C shows the results of 3D-CTAB in the left upper lobe. Figures 8D and Figure 9 (15) show that the course of the intersegmental vein V1+2c between the upper division and the lingula was outside the virtual 3D safety margin, which was 6 cm in diameter. Therefore, we deemed it possible at the preoperative conference to secure a sufficient surgical margin using left upper division segmentectomy. The pulmonary artery and vein to the involved segment (V1+2, V3c, A3a, A1+2c, A1+2a+b+A3b) were resected first. Next, the surgeons identified inflation-deflation line after inflate left upper division and removed using stapling device. Pathologic findings confirmed the diagnosis of well-differentiated adenocarcinoma, and the surgical margin was free of tumor. No recurrence was seen at last follow-up, 4.5 years after surgery.

Discussion
Patients with lung cancer often have respiratory complications such as chronic obstructive pulmonary disease and interstitial lung disease. Furthermore, synchronous or metachronous multiple lung cancers often occur. There are a lot of patients whom limited to surgery have to be selected in order to preserve their respiratory function as shown in this report. For such patients, pulmonary segmentectomy preserves pulmonary function to a greater degree than lobectomy, but the smaller procedure could increase the risk of locoregional recurrence at the resection line (16,17). Therefore, preoperative planning is essential to secure a substantial margin from the primary tumor, especially for VATS segmentectomy. The use of 3D-CTAB with a virtual 3D safety margin helps the thoracic surgeon recognize accurate distances and positional relations between the primary tumor and the intersegmental veins. Most 3D workstations can create a virtual 3D safety margin; it requires only basic workstation functions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Informed Consent: The IRB in our institution waived the requirement for written informed consent from patient.
References
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Akiba T, Marushima H, Harada J, et al. Importance of preoperative imaging with 64-row three-dimensional multidetector computed tomography for safer video-assisted thoracic surgery in lung cancer. Surg Today 2009;39:844-7. [Crossref] [PubMed]
- Oizumi H, Endoh M, Takeda S, et al. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann Thorac Surg 2010;90:1382-3. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Eguchi T, Takasuna K, Kitazawa A, et al. Three-dimensional imaging navigation during a lung segmentectomy using an iPad. Eur J Cardiothorac Surg 2012;41:893-7. [Crossref] [PubMed]
- Chan EG, Landreneau JR, Schuchert MJ, et al. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:523-8. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, et al. Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 2016;8:S710-S715. [Crossref] [PubMed]
- Iwano S, Yokoi K, Taniguchi T, et al. Planning of segmentectomy using three-dimensional computed tomography angiography with a virtual safety margin: technique and initial experience. Lung Cancer 2013;81:410-5. [Crossref] [PubMed]
- Iwano S, Usami N, Yokoi K, et al. Segmentectomy simulation using a virtual three-dimensional safety margin. Ann Thorac Surg 2012;93:e37-9. [Crossref] [PubMed]
- Iwano S, Ito R, Umakoshi H, et al. Evaluation of lung cancer by enhanced dual-energy CT: association between three-dimensional iodine concentration and tumour differentiation. Br J Radiol 2015;88:20150224. [Crossref] [PubMed]
- Iwano S. The 3D-CTAB. Asvide 2017;4:245. Available online: http://www.asvide.com/articles/1554
- Iwano S. Virtual 3D safety margin (blue hemisphere) merged with the 3D-CTAB image. Asvide 2017;4:246. Available online: http://www.asvide.com/articles/1555
- Iwano S. 3D-CTAB view of patient 1. Asvide 2017;4:247. Available online: http://www.asvide.com/articles/1556
- Iwano S. 3D-CTAB with virtual 3D safety margin (blue hemisphere) on the solid nodule in the right lower lobe. Asvide 2017;4:248. Available online: http://www.asvide.com/articles/1557
- Iwano S. 3D-CTAB with virtual 3D safety margin of patient 2. Asvide 2017;4:249. Available online: http://www.asvide.com/articles/1558
- Nomori H, Mori T, Ikeda K, et al. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: a prospective study at a single institute. J Thorac Cardiovasc Surg 2012;144:87-93. [Crossref] [PubMed]
- Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins--implications for patient selection for segmentectomy. Eur J Cardiothorac Surg 2007;31:522-7; discussion 7-8. [Crossref] [PubMed]
Cite this article as: Iwano S. Planning video-assisted thoracic surgery segmentectomy using three dimensional computed tomography angiography and bronchography with a virtual safety margin. J Vis Surg 2017;3:82.


